A 500 KHz localized current field hyperthermia system for use with ophthalmic plaque radiotherapy
Int. J. Hyperthermia, 1987, Vol. 3, No. 5, 423-432, Copyright Taylor & Francis Ltd.
Melvin Astrahan,¹ Peter Liggett,² Z. Petrovich,¹ and Gary Luxton¹
1. Department of Radiation Oncology
2. Department of Ophthalmology
University of Southern California School of Medicine, Los Angeles, California 90033, U.S.A.
Ophthalmic plaque radiotherapy has been demonstrated to be a useful alternative to enucleation in the treatment of small choroidal melanomas. The prognosis for tumours larger than 8 mm in height, however, continues to be poor. Treatment complications limit the radiation dose which may be delivered to these larger tumours. Hyperthermia has been shown to enhance the effectiveness of radiotherapy for many tumours, particularly malignant melanoma. The use of hyperthermia in conjunction with plaque radiotherapy may improve local tumour control for larger choroidal melanomas, allowing patients to maintain useful vision.
We have developed an instrument which enables the combination of localized current field hyperthermia with radiotherapy using an episcleral plaque. The system is simple and inexpensive. We have measured temperature distributions in tissue-like phantoms, in excised bovine eyes, and in vivo in normal rabbits. In each of the cases studied, temperature varied by less than 1°C within 3 mm of, and across the concave surface of the plaque. At distances greater than 3 mm, the temperature gradient was approximately -0.3°C per millimetre.
(Received 10 February 1987; revised 7 May 1987)
1. Introduction
Choroidal melanoma is the most common primary intraocular tumour in adults. Historically, enucleation was the preferred method of treatment for this disease. Zimmerman and McLean (1978,1979) have suggested that enucleation may, in fact, promote the development of metastasic disease. Regardless of this controversy, for patients with tumours exceeding 200 mm³ or 10 mm largest diameter the prognosis for long term survival following enucleation is poor (McLean 1977, Shamma 1977).
Ophthalmic plaque radiotherapy using I-125 and a variety of other isotopes has been demonstrated to be a useful alternative to enucleation in the treatment of small choroidal melanomas (Stallard 1966, Packer and Rotman 1980, Brady et al. 1982, Lommatzsch 1983) and is being used with increasing frequency. This technique permits eradication of the tumour while preserving the eye. While initial tumour responses following plaque radiotherapy have been good, late complications continue to be documented. Complications include cataracts, vasculopathy of the retina and optic nerve, and neovascular glaucoma. These treatment complications result in a significant decrease in visual acuity. The occurrence of late complications limits the radiation dose which may be delivered to larger tumours. In particular, the prognosis for tumours larger than 8 mm in height remains poor.
Hyperthermia has been shown to enhance the effectiveness of radiotherapy for many tumours, particularly malignant melanoma. The thermal enhancement ratio for melanoma is reported as 2.0 (Kim et al. 1982, Gilette 1984, Overgaard 1986). In addition, hyperthermia in conjunction with low-dose-rate irradiation (Harisiadis et al. 1978) has been shown to have the greatest therapeutic potential. The adjunctive use of interstitial hyperthermia with interstitial Ir-192 implants is now practised at many institutions.
The combination of hyperthermia and plaque radiotherapy for the treatment of choroidal melanoma is expected to improve local control of large tumours. It is possible that in the future we may be able to reduce the radiation dose without compromising control of these tumours. Riedel et al. (1985) have shown that ultrasonically produced hyperthermia potentiates the tumouricidal effectiveness of proton beam irradiation for Greene melanoma in the rabbit eye. A lower radiation dose may decrease the incidence of late complications and allow patients to maintain useful vision.
Several techniques have been reported as producing satisfactory ophthalmic heating in experimental tumours. A 4.75 MHz ultrasound system (Riedel et al. 1985) was used to heat tumours in proptosed rabbit eyes. Entry windows for ultrasound beams in humans, however, are more limited. A 2450 MHz microwave stripline applicator has been described (Lagendijk 1982 b). This system heats the entire retina, requires a corneal cooling system, and the patient must be anaesthetized during the hyperthermia procedure. For choroidal melanoma it is unnecessary to heat the entire retina. When combined with plaque radiotherapy, either of these approaches would add one hour to the anaesthesia required for plaque implantation.
A more practical system would include the heating and thermometry devices within the plaque itself. This would reduce the technical complexity of combining hyperthermia with plaque radiotherapy. Since the plaques are sutured in a fixed position adjacent to the tumour, such a system would require no additional localization procedures, and would be tolerant of eye movement. This would permit hyperthermia to be delivered without additional anaesthesia.
Finger et al. (1983,1984,1985,1986) have heated choroidal melanomas (Greene) in the rabbit using an 8 mm diameter, 5.8 GHz microwave antenna built into an episcleral plaque. The temperature gradient produced by their device in a tissue equivalent phantom was about -1°C per mm, with a variation of 2°C across the surface of the antenna. A similar temperature gradient was found in vivo in the rabbit eye. The large temperature gradient of this microwave device severely limits the size and height of tumour which it can heat. In this article we present an alternative approach to delivering hyperthermia from an episcleral plaque using a low frequency technique.
2. Methods
Localized current field (LCF) hyperthermia was selected as the heating modality for this particular application. At a radio frequency (RF) of 500 KHz, as compared to microwave frequencies, tissue behaves more like a conductor than as a dielectric. As a result, electric field attenuation is greatly diminished. At 500 KHz, the 1/exp attenuation depth in tissue is in the order of I metre, compared to about I cm at 5.8 GHz. In addition, the use of a lower frequency simplifies the design of the thermometry system. Inexpensive devices such as thermistors and microthermocouples with metallic leads are much less susceptible to self heating at the lower frequency.
LCF heating results from the power dissipated by radio frequency (RF) currents conducted through tissue between two or more metallic electrodes. The electrodes may be cylindrical (Doss and McCabe 1976, Astrahan and Norman 1982) or planar in form. One arrangement of planar electrodes suggested by Doss and McCabe (1976) involves the use of a small electrode which has been surgically implanted adjacent to a tumour. To complete the circuit, a second electrode of much larger surface area is placed elsewhere on the body. The difference in electrode size results in proportionally greater current density near the smaller electrode. The power dissipated at any point in the tissue by RF currents may be expressed as P = J²r, where P (W/cm³) is the power density, J (A/cm²) is the magnitude of the resistive rms current density, and r (ohm-cm) is the local tissue resistivity. Since heating is proportional to J², significantly greater local heating occurs near the smaller electrode.
The principle of local hyperthermia by the pairing of large and small planar electrodes may be applied to ophthalmic plaque radiotherapy by considering the episcleral plaque to be the small electrode. Heating may be further localized to the concave surface of the plaque-electrode adjacent to the sclera by electrically insulating the 'back' (the convex surface) and sides of the plaque with a thin layer of varnish. This is illustrated diagrammatically in figure 1.
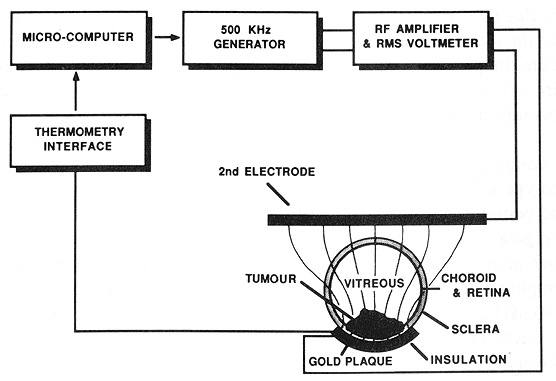
Figure 1. The LCF ophthalmic hyperthermia system in diagrammatic form. A microcomputer controls a 500 KHz sine wave generator and RF power amplifier. Temperature is regulated by feedback from thermocouple sensors mounted on the concave surface of the plaque. The tissue volume adjacent to the concave surface of the plaque is selectively heated because of the increased current density relative to the second electrode.
The heating behavior of plaque-electrodes was evaluated using plaques which did not contain radioactive seeds. First, the effects of selectively insulating a portion of the plaque's surface were studied by observing melting patterns in a tissuelike gelatin phantom (Astrahan 1979 a). This was followed by three-dimensional mapping of the temperature distribution around a plaque. Mapping data were obtained under steadystate conditions in a non-melting tissue-like gelatin phantom (Astrahan 1979 b). Phantom testing was followed by measurements of central-axis temperature distributions in excised bovine eyes and in vivo in rabbit eyes.
2.1. Plaque construction
The LCF plaque-electrode used for the phantom and in vitro studies is illustrated in figure 2. A plaque of the same design, but 5 mm smaller in each axis was used to heat the rabbit eyes. A plaque is cast in gold from a wax model as a 1 - 5 mm thick portion of a 25 mm inner diameter spherical shell. The high atomic number (Z) and high density of the gold provides effective radiation shielding to adjacent organs. The high thermal conductivity of the gold helps to homogenize the local temperature distribution.
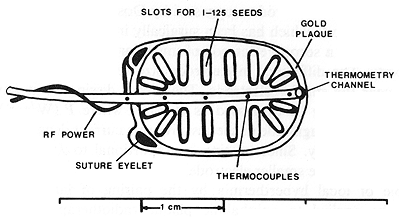
Figure 2. View of the concave surface of an LCF thermo-radiotherapy plaque. The plaque is cast from gold and contains slots into which Ir-192 or I-125, seeds may be glued. The remaining metallic surface of the plaque acts as one electrode of the LCF hyperthermia system. A five sensor microthermocouple array is epoxied into a channel which follows the long axis of the plaque surface. RF power (500 KHz) is supplied to the plaque by a small wire soldered to one of the suture eyelets.
The exact shape and dimensions of the plaque may be adjusted to the particular situation. The edges may be shaped, or notches may be cut to enable placement adjacent to the optic nerve. Eyelets for suturing the plaque to the sclera and for connecting a power wire are made in the edges of the plaque, or may be part of the original casting. One millimeter deep slots for I-125 seeds and a central channel for a microthermocouple array are then machined into the plaque or may also be part of the original casting. Once a basic plaque has been designed, it is easily reproduced using the 'lost-wax' process.
The radioisotope seeds are then glued into the slots using a cyanoacrylate adhesive. Gluing the seeds into a slot insures that the plaque will make mechanically smooth and thus uniform electrical contact with the scleral surface. It is important for the proper functioning of the plaque that the adhesive does not extend beyond the slot and unintentionally insulate the plaque surface, To make the plaque into an electrode, an insulated 30 gauge wire is then soldered to one of the eyelets to supply the RF current and the microthermocouple array is epoxied into the central channel.
2.2. System design
The prototype system illustrated in figure I was used to evaluate the technique. An Apple //e microcomputer (Apple Computer, Cupertino, CA) acts as a system controller for a function generator, a power amplifier and an eight channel thermometry system. The RF power amplifier is an ENI 240L (Electronic Navigation Industries, Rochester, NY). The amplifier provides 50 dB power gain into a 50 ohm load over the range 20 KHz to 10 MHz, and is capable of delivering 100 watts to certain loads. In this design, as a safety factor, the maximum output power of the system is intentionally limited to 30 watts.
An integrated circuit function generator with voltage controlled amplitude supplies a 500 KHz since wave to the power amplifier. The maximum output of the function generator was adjusted to deliver 27 watts to a 50 ohm resistor connected across the output of the power amplifier. The function generator output is linearly adjustable in 1 percent increments by the computer software using a digital to analog converter (DAC). A back-up temperature-limiting circuit using a simple relay controls the output of the function generator as an additional safety factor.
An eight-channel thermometry interface was designed using custom thermocouple modules (SensorTek Inc., Clifton, NJ) and DAISI (tm) (Interactive Structures, Bala Cynwood, PA) analog interface cards. Microthermocouple arrays (SensorTek) with three to five sensors separated by two to five millimetres and 29 gauge needle microprobes (SensorTek) are used to measure temperature. The needle microprobes were modified by adding a thin layer of insulating varnish to the needle shaft to enable their use in the RF field. Temperature acquisition and control software for the computer was written entirely in assembly language to assure the most rapid execution speed.
2.3. System performance
The performance characteristics of the system were tested to determine if the 30 watt design would provide sufficient power in clinical operation. The ENI-240L RF power amplifier is designed to drive a 50 ohm load. The actual load experienced by the amplifier during ophthalmic heating could vary considerably from 50 ohms. To determine the amplifier loading under operational conditions, the function generator output is momentarily adjusted to 40 per cent and the rms voltage read from a meter connected across the power amplifier output. (The rms voltmeter is built into the amplifier chassis.) The equivalent resistive load is then determined from figure 3 which relates resistive load to the rms voltage across the load for fixed values of function generator output.
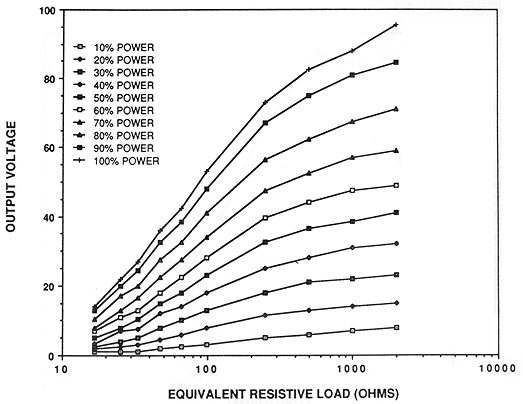
Figure 3. The rms voltage measured across the output of the power amplifier may be used to estimate the loading of the amplifier from this family of curves. Each curve plots the unique relationship of rms voltage to load (simulated by resistors) for fixed function generator output. The function generator output level is controlled by the computer software.
Using this procedure, the equivalent resistive load was found to depend upon the distance between the plaque and the second electrode. For rabbits, placing the second electrode (an approximately 20 cm² piece of copper screen and a saline-loaded sponge) nearby on the shaved forehead resulted in about 120 ohm load, whereas placing the second electrode on the inner surface of an ear resulted in a load in the order of 500 ohms. Using two 'second' electrodes in parallel, one on each ear, reduced the load to about 200 ohms. Excised bovine eyes typically behaved as 70 to 100 ohm loads when the second electrode was placed directly on the eye opposite the plaque. The gelatin phantoms measured 100 to 120 ohms depending on the position of the second electrode.
To drive loads other than 50 ohms could require a load-matching transformer (at additional expense) on the output of the amplifier. To determine if a transformer was required (it was found not to be necessary), resistors were used to simulate the range of loading conditions expected in clinical use. With no transformer, the maximum output power of 28 watts was delivered to an 100 ohm load. Maximum output power was found to fall off rapidly for loads less than 50 ohms, to under 15 W for a 16,5 ohm load. For loads greater than 100 ohms, power falls off gradually to about 15 W at 500 ohms, and to under 5 W at 2000 ohms. This is illustrated in figure 4.
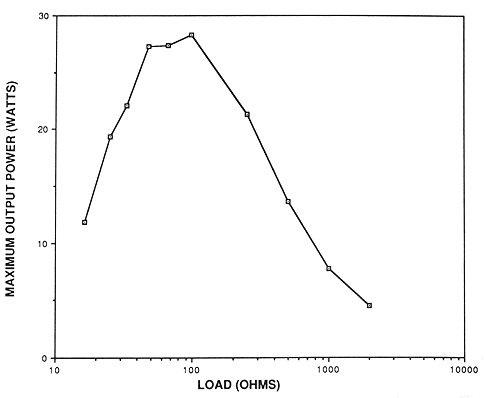
Figure 4. Maximum output power of the system as a function of amplifier loading conditions. The system is capable of delivering more than 15 watts to loads ranging from 25 to 500 ohms. This was experimentally determined to suffice for the range of operating conditions expected in clinical use.
According to the data of figure 4, more than 15 watts of power is available over the load range 25 to 500 ohms. We have experimentally determined that less than 10 watts is necessary to provide rapid heating and good temperature control in the rabbits, excised bovine eyes and various phantoms. These results indicate that adequate power is available over the range of load conditions likely to be encountered, and thus no loadmatching transformer is necessary.
The RF system hardware and software were tested for toxicity in-vivo, using needle electrodes in rats and plaques in rabbits. No adverse physiological effects associated with the frequency, RF power system, or temperature regulating system were found. Temperature at the plaque surface can be maintained at ±0.1°C.
3. Results
Using the melting gelatin phantom technique, we observed that melting (and thus heating) adjacent to insulated surfaces is suppressed compared to uninsulated surfaces. When the plaque is uninsulated, the gelatin melts on all sides of the plaque. When the 'back' side of the plaque is coated with an insulating varnish, only the gelatin in 'front' of the uninsulated side melts.
In all cases (phantom, in vitro and in vivo,) the temperatures measured on the plaque surface either by the multisensor array or by the scanning needle microprobe were slightly greater towards the edges of the plaque, but varied overall by not more than 0.5°C.
The temperature distribution in 'front' of the concave surface of the plaque illustrated in figure 2 was measured in a large, non-melting, tissue-like gelatin phantom (Astrahan 1979), using an insulated 29 gauge needle microprobe. Temperatures were measured under steady-state conditions on a 1 x 2 mm grid in two mutually orthogonal planes whose intersection is the geometric central axis of the plaque.
Figure 5 shows relative temperature distributions measured above half of the long axis of the plaque for two different positions of the second electrode. Isotherms have been referenced to the temperature measured on the central axis by the multisensor array built into the plaque. The left side of figure 5 (phantom 1) illustrates the temperature distribution measured when the second electrode was placed 70 mm away from and lateral to the plaque. The right side of the figure (phantom 2) illustrates the temperature distribution obtained when the second electrode (made of copper mesh) was placed 30 mm directly above the plaque.
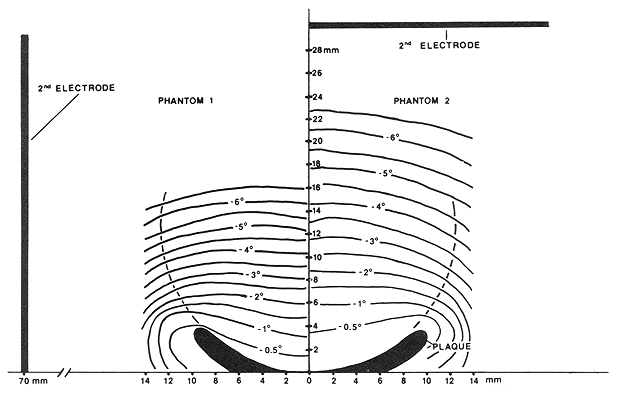
Figure 5. Temperature isotherms measured under steady-state conditions in the plane containing the central axis and the long axis of the plaque surface for two different positions of the second electrode. All temperatures have been referenced to the temperature of the plaque near the central axis. In 'phantom 1', the second electrode was placed 70 mm lateral to the plaque. In 'phantom 2', the second electrode was placed 30 mm directly above the plaque.
Figure 6 plots central-axis temperature distributions measured under steady-state conditions in the two gelatin phantoms discussed previously, in two excised bovine eyes and in an in vivo rabbit eye. Temperatures are referenced to the temperature of the plaque surface. In each case the plaque temperature was raised to about 8°C above the ambient temperature of the object being heated. The data plotted for the gelatin phantoms and for one of the bovine eyes (in vitro no. 1) were obtained using the 29 gauge microprobe. Data in the second bovine eye (in vitro no. 2) were obtained using a five-sensor microthermocouple array (5 mm spacing between sensors). Data from the rabbit eye were obtained in vivo using a five sensor array (2 mm spacing between sensors).
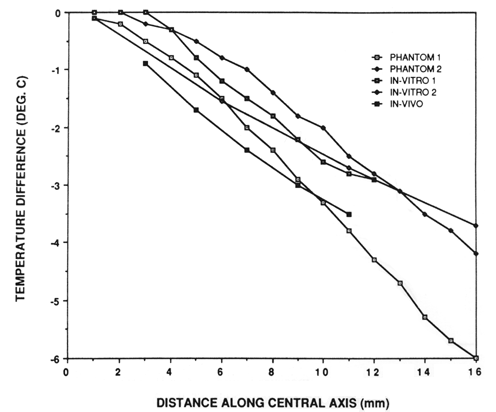
Figure 6. Temperature distributions measured under steady-state conditions on the central axis of the plaque for the two gelatin phantom experiments described in figure 5, two excized bovine eyes and one in vivo rabbit eye.
In the multisensor array used in the rabbit eye measurement, the sensor closest to the plaque surface was itself about 2 mm from the physical end of the probe, which was, in turn, touching the inner surface of the retina. The distance of this sensor was therefore estimated to be 3 mm from the plaque surface.
4. Discussion
The uniformity of temperatures measured across the surface of the plaques suggests that a single thermocouple sensor may suffice for a clinical device. The best position to measure the average plaque temperature appears to be midway between the central axis and the edge of the plaque to which the power wire is attached. This would permit the plaque to hold more radioisotope since the central channel would not have to extend the length of the plaque.
It is apparent from figure 5 that the position of the second electrode can be used to influence the resulting temperature distribution. When the second electrode is far away, the central axis temperature gradient is greater than when the second electrode is nearby. In addition, lateral displacement of the second electrode may skew the temperature distribution slightly in the direction of the second electrode as suggested by the -0.5°C isotherms.
Figure 5 also suggests that therapeutic temperatures may extend to a margin of a few millimetres beyond the edge of the plaque. The plaque used for these measurements was not fully insulated on the convex side. Insulating the edge as well as the 'back' of the plaque may prove useful in reducing any directional effects associated with the second electrode by confining the current to flow from the concave surface. In addition, selectively insulating portions of the concave surface may help to reduce temperatures in nearby critical structures such as the optic nerve. The high thermal conductivity of the gold, however, is expected to mask any effects of insulating small areas of the plaque surface.
Figure 6 suggests that phantom measurements may, in fact, be useful in predicting in vivo temperature distributions for the eye plaques. The phantom and in vitro data along the central axis are generally within 1°C of the in vivo measurements, and the gradient of about -0.3°C per mm between 3 and 11 mm is similar in all cases. In addition, the temperature of the plaque surface is consistently the maximum temperature measured. This suggests that temperatures measured on the plaque surface alone may provide sufficient information to permit safe clinical application. The combination of temperature measurement on the plaque surface with a thermal model of the eye (e.g. Lagendijk 1982 a), and a heating and radiation dosimetry model for the plaques (Luxton et al. submitted for publication) can provide a thermoradiotherapy dosimetry capability. Such a system is currently under development for clinical application of the combined modalities.
In the normal rabbit, the central-axis temperature distribution showed only a 3.5°C decrease at a height of 11 mm above the plaque surface, and less than 0.5°C variation across the plaque surface. This suggests that in the rabbit, heating the plaque to 45°C should result in therapeutic temperatures to distances of 10 mm from the plaque surface. Riedel et al. (1985) reported markedly increased complications for temperatures above 45°C for ultrasonic heating. According to Finger et al. (1984,1985,1986), plaque temperatures up to 47°C may be tolerated.
The physiology of blood flow in the eye (Adler 1975) suggests that hyperthermia delivered by an episcleral plaque may have an additional advantage relating to temperature uniformity. Blood flow in the choroid is reported to be much greater than in any other tissue of the eye. Thus, the normal tissue with the greatest capacity for perfusive cooling is coincidentally at the site of greatest power deposition.
The low cost and operational simplicity of this device should enable a rapid progression from the laboratory to clinical application. The simplicity of plaqueelectrode construction makes a wide variety of custom shapes and sizes possible. The ability to control current patterns near the plaque surface by judicious use of insulation suggests that some selective reduction in temperature to adjacent critical structures such as the optic nerve may be possible. This concept is currently under investigation as is the effect on temperature distribution of the presence of radioisotope seeds in the slots. Preliminary investigations indicate that I-125 seeds have no measurable effect on the temperature distribution. In addition, a major effort is being made to study the acute and late toxic effects of the combined hyperthermia-plaque procedure.
Future enhancements to the LCF instrumentation may include multiplexed power/thermometry capability, and a concentric, dual electrode plaque in which the second electrode is part of the plaque itself.
References
- ADLER'S PHYSIOLOGY OF THE EYE, CLINICAL APPLICATIONS, 1975, 6th edn, edited by R. A. Moses (St. Louis: C. V. Mosby Company) pp. 216-218.
- ASTRAHAN, M. A., 1979 a, Hyperthermia phantom. Medical Physics, 6, 72.
- ASTRAHAN, M. A., 1979 b, Concerning hyperthermia phantom. MedicalPhysics, 6, 235.
- ASTRAHAN, M. A., and NORMAN, A., 1982, A localized current field hyperthermia system for use with Ir- 192 interstitial implants. Medical Physics, 9, 419-424.
- BRADY, L. W., SHIELDS, J. A., AUGSBURGER, J. J., and DAY, J. L., 1982, Malignant intraocular tumors. Cancer, 49, 578-585.
- DOSS, J. D., and MCCABE, C. W., 1976, A technique for localized heating in tissue: an adjunct to tumor therapy. Medical Instrumentation, 10, 16-20.
- FINGER, P. T., PACKER, S., KISTNER, L. M., PAGLIONE, R. W., ANDERSON, L. L. SVITRA, P. P. and Kim, J. H., 1986, A thermoradiotherapy technique for choroidal melanoma. Endocurietherapy, Hyperthermia, Oncology, 2, S33-S37.
- FINGER, P. T., PACKER, S., SVITRA, P., PAGLIONE, R. W., ALBERT, D. M., and CHESS, J., 19 83, A 5.8 GHz ophthalmic microwave applicator for treatment of choroidal melanoma. IEEE MTT-S International Microwave Symposium Digest, F-1, 177-179.
- FINGER, P. T., PACKER, S., SVITRA, P. P., PAGLIONE. R. W., ANDERSON, L. L., Kim, J. H., and JAKOBIEC, F. A., 1985, Thermoradiotherapy for intraocular tumors. Archives of Ophthalmology, Hyperthermia, Oncology, 103, 1574-1578.
- FINGER, P. T., PACKER, S., SVITRA, P., PAGLIONE, R. W., CHESS, J., and ALBERT, D. M., 1984, Hyperthermic treatment of intraocular tumors. Archives of Ophthalmology, Hyperthermia, Oncology, 102, 1477-1481
- GILLETTE, E. L., 1984, Clinical use of thermal enhancement and therapeutic gain for hyperthermia combined with radiation of drugs. Cancer Research (Suppl.), 44, 4836s-4841s.
- HARISIADIS, L., SUNG, D., KESSARIS, N., and HALL, E. J., 1978, Hyperthermia and low doserate irradiation, Radiology, 129, 195-198.
- KIM, J. H., HAHN, E. W., and AHMED, S. A., 1982, Combination hyperthermia and radiation therapy for malignant melanoma. Cancer, 50, 478-482.
- LAGENDIJK, J. J. W., 1982 a, A mathematical model to calculate temperature distributions in human and rabbit eyes during hyperthermic treatment. Physics in Medicine and Biology, 27,1301-1311.
- LAGENDIJK, J. J. W., 1982 b, A microwave heating technique for the hyperthermic treatment tumors in the eye, especially retinoblastoma. Physics in Medicine and Biology, 27, 1313-1324,
- LOMMATZSCH, P. K., 1983, β-irradiation of choroidal melanoma with Ru-106/Rh-106 applicators. Archives of Ophthalmology, 101, 713-717.
- LUXTON, G., ASTRAHAN, M. A., LIGGETT, P., NEBLETT, D. L., and PETROVICH, Z., 1987, Dosimetric calculations and measurements of gold plaque ophthalmic irradiators using Iridium-192 and Iodine-125 seeds. International Journal of Radiation Oncology, Biology, Physics (in the press).
- MCCLEAN, 1. W., FOSTER, W. D., and ZIMMERMAN, L. E., 1977, Prognostic factors in small malignant melanomas ofchoroid and ciliary body. Archives of Ophthalmology, 95,48-58.
- OVERGAARD, J., 1986, The role of radiotherapy in recurrent and metastatic malignant melanoma: a clinical radiobiological study. International Journal ofRadiation Oncology, Biology, Physics, 12, 867-872.
- PACKER, S., and ROTMAN, M., 1980, Radiotherapy of choroidal melanoma with iodine-125. Ophthalmology, 87, 582-590.
- RIEDEL, K. G., SVITRA, P. P., SEDDON, J. M., ALBERT, D. M., GRAGOUDAs, E. S., KOEHLER, A. M., COLEMAN, D. J., TORPEY, J., Lizzi, F. L., and DRILLER, J., 1985, Proton beam irradiation and hyperthermia, effects on experimental choroidal melanoma. Archives of Ophthalmology, 103, 1862-1869.
- SHAMMA, H. F., and BLODi, F. C., 1977, Prognostic factors in choroidal and ciliary body melanomas. Archives of Ophthalmology, 95, 63-69.
- STALLARD, H. B., 1966, Radiotherapy for malignant melanoma of the choroid. British Journal of Ophthalmology, 50, 147-155.
- ZIMMERMAN, L. E., and McLEAN, I. W., 1979, An evaluation of enucleation in the managment of uveal melanomas. American Journal of Ophthalmology, 87, 741-760.
- ZIMMERMAN, L. E. McLEAN, I. W., and FOSTER, W. D., 1978, Does enucleation of the eye containing malignant melanoma prevent or accelerate the dissemination of tumor cells. British Journal of Ophthalmology, 62, 420-425.
Plaque Simulator References |
Guide Contents





