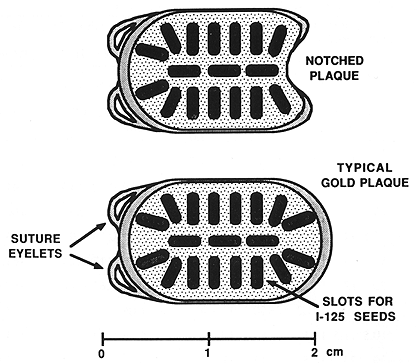
Figure 1. One of the episcleral gold plaques that was used in the treatment of patients with choroidal melanoma.
Between 1983 and 1987, seventeen patients with large primary malignant melanoma of the choroid were treated with episcleral radioactive plaque. All patients refused enucleation. Mean tumor dimensions were as follows: height, 10.5 mm; length, 15.1 mm; and width, 14.0 mm. Treatment was given with iridium-192 in 11 patients (65%) and iodine-125 in six patients (35%). Mean radiation dose at 5 mm was 225 Gy; at the tumor apex, 75 Gy. Mean follow-up was 33 months. All patients were alive, including two with metastatic disease. Of the 17 patients treated, 16 (94%) had objective tumor regression with a mean decrease in tumor height of 60%. Tumor regression did not correlate well with radiation dose. Visual acuity was improved in two patients, unchanged in eight, and decreased in seven. Acute complications were of minor significance; however, late complications presented a major problem. Five patients required enucleation, including four for late complications and one for progressive disease. Enucleation correlated well with the initial tumor elevation (P<.001).
Improved treatment methods are needed to address the problem of patients with T3 malignant melanoma of the eye. Adjuvant hyperthermia is currently being investigated in an attempt to increase tumor control and lower the incidence of late complications.
Key Words: T3, Large, Malignant Melanoma, Eye, Episcleral PlaquePrimary malignant melanoma of the eye is an uncommon tumor representing less than 10 % of all malignant melanomas (1). In 1988, about 1600 new cases are expected to be diagnosed in the United States (1). The natural history of this tumor is that of a slow local growth, with invasion of the orbital structures and metastatic disease, primarily to the liver, being relatively late events (2-6). Surgical treatment of this tumor, with few exceptions, requires enucleation (7,8). Episcleral radioactive plaque therapy provides a useful alternative to the surgical treatment. It offers survival rates equal to those of surgery with a major benefit, which is the preservation of function of the eye in the majority of patients (9-12). Similar good treatment results, with preservation of vision, can be obtained using particle beam therapy such as helium or proton beams (13-15). These good treatment results are primarily limited to tumors less than 8 mm in height or T1 and T2 lesions (9,16). Larger tumors (T3), particularly those of more than 8-mm elevation, with few exceptions require surgical treatment for a good chance of control. The main reason for this is the need for higher doses of radiation required to control larger tumors. These higher doses of radiation are more likely to result in an increased incidence of complications compromising eye function.
The purpose of this report is to present our experience with episcleral radioactive plaque therapy in patients with large tumors and who refuse enucleation.
From June 1983 through June 1987, fifty-six patients with primary malignant melanoma of the choroid were treated with episcleral radioactive plaque at the University of Southern California (USC) School of Medicine in Los Angeles, California. Of the 56 patients, 39 (70%) received elective episcleral radioactive plaque while 17 (30%) with large (T3) tumors (more than 8 mm in elevation) refused recommended enucleation and were treated with episcleral radioactive plaque. There were ten female and seven male patients. Their ages ranged from 48 to 79 years, with a mean age of 66 years.
Signs and symptoms at presentations were retinal detachment in 11 (65%), decreased vision in six (35%), photopsia in three (18%), and vitrous hemorrhage in one (6%). Four patients had two signs or symptoms at diagnosis. Pretreatment workup of patients was directed toward the exclusion of metastatic disease and toward defining and diagnosing the primary tumor. All patients had detailed history and physical examination. Radiographic examination consisted of a chest film with computed tomography, used only if there was an indication of possible abnormality obtained from history, physical examination, or laboratory tests. These tests included complete blood cell count, serum alkaline phosphatase, SGOT, and SGPT. Ophthalmological examination included biomicroscopy, providing detailed information on tumor characteristics and location, visual acuity, and ultrasound examination using A and B scans, which provided tumor dimension data and its characteristics.
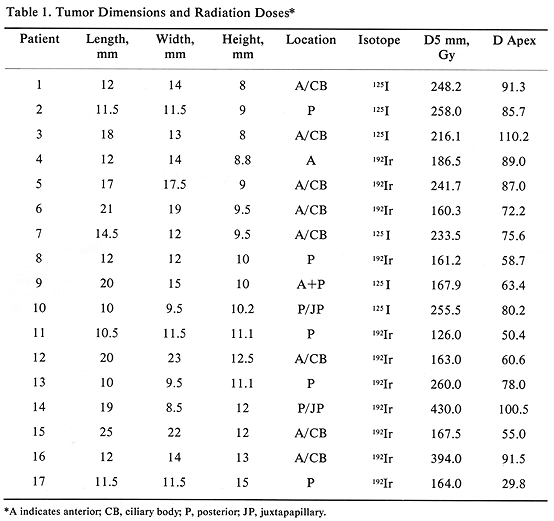
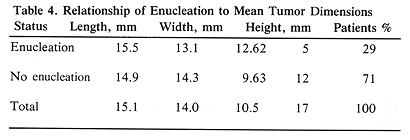
All 17 patients had large tumors staged as T3 (17). The tumor height ranged from 8 to 15 mm, with a mean of 10.5 mm. The largest tumor dimension (length) ranged from 10 to 25 mm (mean, 15.1 mm) and the remaining dimension (width) ranged from 8.5 to 23 mm (mean, 14.0 mm) (Table 1). The tumor was located posteriorly in eight patients, including two with juxtapapillary location, and one large tumor extending anteriorly (Table 1). The remaining ten patients had tumors located anteriorly. Episcleral plaques used in this study were made of gold, designed and custom-built at USC by Luxton and Neblett (Figure 1). Detailed descriptions of these plaques and radiation dosimetry have been published.11,19 Radioactive isotopes used for the treatment of these 17 patients included iridium-192 in 11 (65%) and iodine-125 in six (35 %) patients. At this time, radioactive I-125 is used for the treatment of all patients. Radiation doses were prescribed at the tumor apex and at 5-mm depth. D5 was defined as tumor dose 5 mm from the outside surface of the sclera. The D5 doses ranged from 126.0 to 430.0 Gy (mean, 225.5) (Table 1).
Episcleral plaque placement was performed with the patient under local anesthesia. Plaque position was optimized using intraoperative transillumination. The plaque was secured in place with sutures attached to the sclera. The average treatment was completed in seven days and was administered on an outpatient basis.
Follow-up extended from 11 to 57 months, with a mean of 33 months. The first follow-up examination was scheduled one month after the completion of treatment. During the first posttreatment year, the frequency of follow-up examinations was three months, which was extended to six months in the second posttreatment year. All of the pretreatment tests were performed at each follow-up with the exception of a chest radiograph, which was obtained annually.
At the time of this analysis, all 17 patients were alive, including two with metastatic disease. Patient 6 had tumor dimensions at diagnosis of 20 X 23 X 12.5 mm and developed skin metastases at 22 months. Patient 12 with a 21 x 19 x 9.5 mm tumor had liver metastases at 17 months.
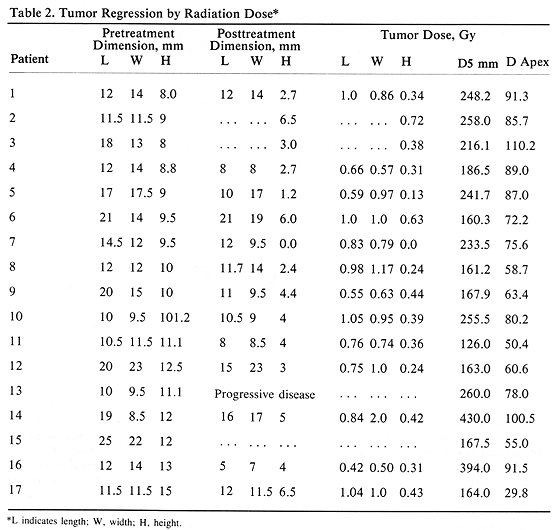
Tumor regression was observed in 16 patients (94%), while one patient (6%) with a 10 X 9.5xll.l-mm tumor and D5 mm-260 Gy had tumor progression. The degree of tumor regression, measured with ultrasound, did not correlate well with radiation dose (D5 mm and D apex) (Table 2). The ratio of the posttreatment tumor height to the pretreatment height ranged from 0 to 0.72 with a mean ratio of 0.36 (Table 2). It is of interest to note a better degree of tumor regression recorded in tumor height than in the tumor length or width (P<.01), Bonforroni method of comparing multiple means, Table 2). Analysis of posttreatment to pretreatment height ratio showed one patient with value greater than 1.0 and 12 patients with the ratio less than 0.50. This compared with five patients having tumor length ratio greater than 1.0 and only one patient with the ratio less than 0.50. A similar poor ratio was seen comparing posttreatment to pretreatment tumor width with the tumor height (Table 2). The posttreatment tumor dimensions were not recorded in one patient, with two additional patients missing posttreatment width and length.
Visual acuity improved following treatment in two patients, remained unchanged in eight, and decreased in seven. Of the seven patients with decreased visual acuity, three had a minor decrease, one had a decrease from 2/200 to light perception only, while three patients had no light perception.
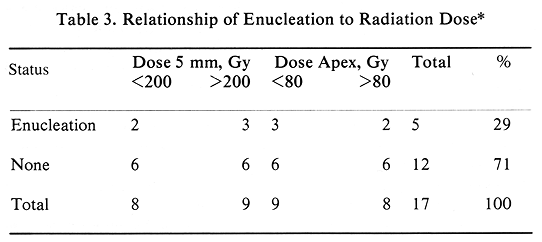
Episcleral plaque therapy has been very well tolerated by these 17 patients. Acute treatment toxicity consisted of minor problems requiring no medical treatment. As expected of this patient population, there has been a major late treatment toxicity. Enucleation was performed in five patients (29%). Of these five patients, one had progressive tumor, and four required enucleation for complications. These complications included one rubeosis, one scleral thinning, and one rubeosis with scleral necrosis; in one patient this procedure was performed outside of USC and no information on indications for surgery was available.
The initial tumor elevation was an important parameter predicting enucleation. The mean tumor height of enucleated patients was 12.62 mm as compared with the mean of 9.63 mm for the remaining patients (P=.001, t-test). All five patients requiring enucleation had this procedure performed within 22 months of therapy (Figure 2). The mean time to enucleation in these five patients was 11.6 months (Figure 2). Among the 12 patients who had no enucleation, two had no late complications, four had retinal detachment (which subsequently resolved), three had vitrous hemorrhage (it resolved in two patients), one had radiation retinopathy, one had rubeosis, and one had neovascular glaucoma. This group of patients with late complications included one with retinal detachment, who also developed a cataract, which required surgical removal at eight months. The four patients who had retinal detachment are difficult to evaluate, since they had this problem prior to episcleral plaque therapy.
Management of the patients with large primary malignant melanomas of the choroid who refused enucleation presents a therapeutic dilemma. Surgical treatment is an effective therapy to control the tumor, but it requires removal of the eye, which may create difficult emotional problems in some patients. We estimate this problem to affect approximately one third of patients who present with tumors requiring enucleation. Episcleral radioactive plaque therapy is an effective alternative to enucleation, particularly for small and medium lesions. In larger tumors brachytherapy may result in substantial tumor shrinkage as evidenced by 16 (94%) of our patients showing a decrease in tumor dimensions. of which 13 (76%) had more than a 50% decrease in tumor elevation. These patients, however, experienced a high incidence (23%) of late toxicity requiring enucleation.
Treatment outcome and the incidence of late toxicity in our patients are similar to those reported in the literature for the same patient population (10,12,16,20,21). In one report, however, patients treated with proton beam teletherapy experienced fewer complications (14).
In an attempt to optimize radiotherapy for larger choroidal tumors, the use of adjuvant hyperthermia has been under study. Hyperthermia is expected to enhance antitumor activity of radiotherapy and, if proven effective, may result in a reduction of brachytherapy dose. This in turn is expected to reduce late complications of irradiation. A successful preclinical study on the use of proton beam irradiation with adjuvant 4.75-MHz ultrasound hyperthermia has been reported by Riedel et al. (22) Finger et al. (23) reported using I-125 brachytherapy combined with 4800-MHz microwave hyperthermia, and Lagendijk (24) has developed a useful ocular hyperthermia system using a 2450-MHz instrument. A recent USC report presented a preclinical study of a localized current field (500 KHz) hyperthermia system using a modified radioactive I-125 episcleral plaque (25). This system was proven safe in terms of both acute and late toxicity in experimental animals using temperatures of up to 44°C. A phase I clinical study is currently under way at USC. Results of phase I studies conducted in several medical centers are expected in the near future.