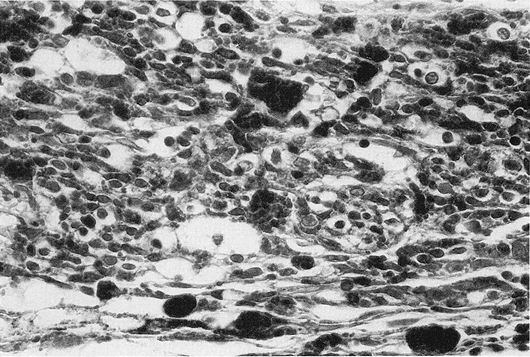
FIG. 1. Individual cell necrosis, reflected by pigment laden macrophages in an eye treated with episcleral radioactive plaque therapy (RPT). (Hematoxylin and eosin; X200, 86-1428.)
Histopathologic studies were performed on 38 eyes in patients with uveal melanoma who had enucleation. Of the 38 eyes examined, enucleation was required in 11 (29%) following episcleral radioactive plaque therapy (RPT), which was performed in 83 patients. The reasons for enucleation in the 11 patients who had RPT were progressive tumor in 5 and treatment complications in 6 eyes. The histologic findings in these 11 patients were compared to those seen in 7 patients (18%) who received a planned course of preoperative external beam radiotherapy (RT) prior to enucleation and with 20 uveal melanoma patients (53%) who were treated with enucleation alone. Tumor necrosis was found in the eyes of patients from all three groups examined. It was, however, seen more frequently and to a greater extent in the 11 RPT patients as compared to the 7 preoperative RT and 20 enucleation alone patients, p = .01. There was no difference in the incidence or extent of tumor necrosis in the 7 preoperative RT patients as compared to the 20 primary enucleation patients, p = .18. In all 3 study groups, no correlation was found between tumor size and necrosis. In the 11 RPT patients, necrosis was independent of cell type and the radiation dose. As expected, the RPT patients had a greater incidence of neovascularization on the iris and scleral necrosis than those of the other two study groups (70 vs. 12.5% and 33 vs 0%, respectively), p = .004. A major effort needs to be made to optimize episcleral RPT in order to reduce treatment complications and increase the incidence of primary tumor control.
This work was supported in part by the National Institutes of Health NEI Core Grant for the Histopathology Laboratories.
Radioactive episcleral plaque therapy (RPT) is an important primary treatment modality in the management of patients with primary uveal melanoma (1). In the past decade it has become a recognized treatment alternative to enucleation. In the largest series reported on the treatment of patients with uveal melanoma this increasing importance of RPT has been clearly demonstrated (1).
In the past 15 years, several reports have been published on the pathologic examination of eyes treated with RPT or particle beam irradiation (2-5). The eyes examined required enucleation, either for tumor progression or for severe complications of therapy. In the late 1970s a hypothesis was made on the increase in the incidence of metastatic disease in uveal melanoma patients following enucleation (6). This hypothesis stimulated an interest in employing measures to prevent metastatic disease in patients treated with surgery. Preoperative irradiation has been widely used since the early 1980s with the aim to accomplish this goal. Pre or postoperative radiotherapy has been proven effective in preventing local tumor recurrence and in decreasing the incidence of metastatic disease in several extraocular primary tumors. In the absence of prospective randomized trials this effectiveness of adjuvant radiotherapy cannot be proven at this time in patients with posterior uveal melanoma.
The aims of this report were to examine the histopathologic findings in uveal melanoma patients who required enucleation following RPT and to compare these findings with those seen in eyes following preoperative external beam radiotherapy and enucleation alone.
Between August 1983 and December 1989, 212 patients with uveal melanoma were evaluated at the Estelle Doheny Eye Medical Clinic of the University of Southern California School of Medicine. Of these 212 patients, 83 (39%) were treated with RPT, 7 (3.3%) received a course of external beam radiotherapy prior to enucleation, 8 (3.8%) were treated with episcleral plaque thermoradiotherapy, and 13 (6.1 %) were treated as a part of the Collaborative Ocular Melanoma Study (COMS). The remaining 101 patients (47.6%) had either enucleation alone (86 patients) or had small tumors (15 patients) that were observed and not further treated.
For the purpose of this study, patients were categorized into one of the following three groups (7) according to tumor size at the time of pretreatment ophthalmologic evaluation: (a) small (thickness <= 3 mm and basal diameter <= 5 mm); (b) intermediate (thickness 3.1-8 mm and basal diameter <= 16 mm); and (c) large (thickness > 8 mm or basal diameter > 16 mm or both).
The 83 patients managed with RPT were treated with USC-designed and built 18K gold plaques. Prior to 1986, patients received their treatment with Iridium-192, while Iodine-125 was used for treatment in the subsequent years. Details on the treatment techniques, radiation dosimetry, treatment optimization techniques, plaque design, survival, and complications have been previously published (8-11). Of the 83 patients treated with RPT, 11 (13%) required enucleation. The mean time from treatment to enucleation was 14.5 months (range: 3-27 months). Complications of RPT, including neovascular glaucoma, intraocular hemorrhage, and radiation retinopathy, resulting in a painful, nonfunctioning eye, led to enucleation in 6 of these 11 patients. In 4 of the 6 enucleated eyes there was a 75% decrease in the tumor's apical dimension. This tumor height decrease was recorded by ultrasound. Progressive growth of primary tumor necessitated enucleation in 5 patients.
Patients selected for this study included all 11 patients who required enucleation following RPT, all 7 patients who received a course of preoperative irradiation prior to enucleation, and 20 randomly chosen patients who were treated with enucleation alone. No patient who participated in COMS trials or received episcleral plaque thermoradiotherapy was part of this study.
In the 83 RPT patients radiation doses were defined at D5mm and at the tumor apex (Dapex). A mean D5mm was 150.1 Gy (range: 70.5-430 Gy), while it was 102.6 at Dapex (range: 29.6-200 Gy). In the group of 11 RPT patients who required enucleation, a mean D5mm was 206.3 Gy (range: 125-430 Gy).
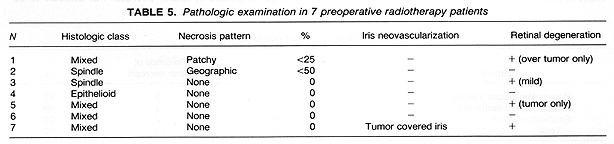
External beam radiotherapy prior to enucleation was given in 7 patients. The treatments were administered with a linear accelerator-generated 4-MV photon beam. Two radiation portals were used for the treatments including anterior and a 40° angled, lateral fields toward the occiput, with 60° wedges. The total radiation dose was 20 Gy, given in 5 equal daily fractions of 4 Gy each. The radiation dose was to encompass the orbital content and was defined at the isocenter intersection. The dose inhomogeneity was ±4%. The 7 patients who had this preoperative external beam radiotherapy underwent enucleation within 72 hours of the completion or irradiation.
The distribution of patients by tumor stage and treatment group is shown in Table 1. No small tumors were treated. As expected, there were more patients with intermediate tumors treated with RPT than with enucleation alone (45 vs 35%). No intermediate tumor group patients were treated with preoperative external beam radiotherapy. Although enucleation was the treatment of choice for large tumors, patients who refused enucleation were offered RPT.
The enucleation specimens were formalin-fixed, many after intraoperative sectioning in the fresh state. Gross and histopathologic examinations were performed on each globe. Whenever possible, complete cross sections, including the pupil, tumor, and optic nerve head, were evaluated. Some cases had 2 to 3 sections available, but for most a single slide was available for examination. When specific structures, such as the iris, were absent or insufficient for evaluation, these cases were not included in comparative statistics pertaining to the relevant feature, such as neovascularization on the iris.
Histopathologic evaluation was done jointly by a pathologist and a radiation oncologist without knowledge of the treatment group. Slides were evaluated three separate times and discrepancies or disagreements between examiners were resolved. The following parameters and features were identified on hernatoxylin and eosin-stained sections:
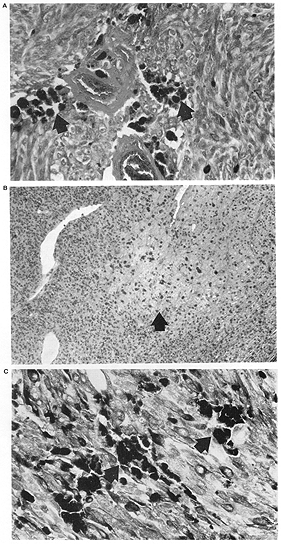
FIG. 2. A: Patchy necrosis with islands of necrotic cells (arrows) RPT. B: Preoperative external beam radiotherapy; C: Enucleation alone eye. (Hematoxylin and eosin; Fig. A: X200, 87-781; Fig. B: X200, 86-1248; Fig. C: X200, 82-1391.) |
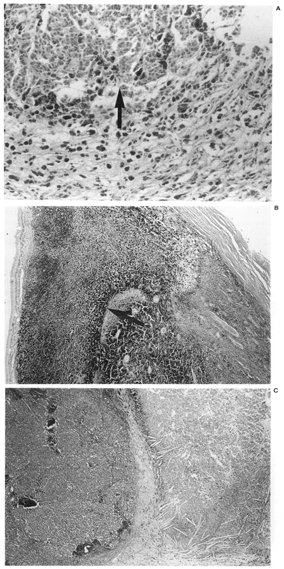
FIG. 3. Geographic tumor necrosis. A: Less than 50% of this preoperative external beam radiotherapy treated eye is necrotic, with viable cells (arrow) at the edge of the field. B: This eye treated with radioactive plaque therapy had greater than 50% tumor necrosis (arrow). C: An eye with enucleation alone also exhibited greater than 50% necrosis, and the necrotic areas contained cholesterol clefts (Hematoxylin and eosin; Fig. A: X200 87-154; Fig. B: X20, 85-179; Fig. C~ X20, 86-027.) |
We investigated tumor necrosis, using either presence or absence of necrosis or tumor comprised of <50% or >50% necrosis as the outcome, for each of the treatment groups and as well as by the tumor size, cell type, and radiation dose levels. Standard methods for contingency tables were used to assess the statistical significance of the differences in tumor necrosis over each of these factors. The occurrence of adverse outcomes, such as scleral necrosis or neovascularization on the iris, were investigated in a similar manner. When it seemed warranted, the contingency tables were collapsed into 2 X 2 tables, in which case Fisher's exact test was used. Variations were considered significant if they achieved a 95% (two-sided) significance level.
Histologic examination of tumors from the 38 enucleated eyes revealed fewer spindle cell, mixed cell, and epithelloid lesions in the 11 RPT patients as compared to those who received preoperative RT or were treated with enucleation alone (NS) (Table 2). The 11 RPT patients had three necrotic-type lesions, while none of the 27 patients from the other two treatment groups had this histologic type, p = .02 (Table 2).
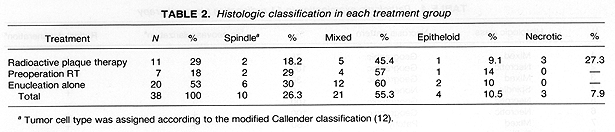
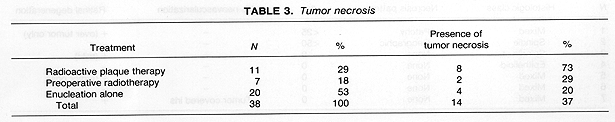
Tumor necrosis was identified in all three treatment groups. It was present most frequently in the 11 eyes of patients treated with RPT as compared to those patients who received preoperative radiotherapy or no radiotherapy 8/11 (73%) vs 6/27 (22%), p = .006 (Table 3). The pattern of necrosis was predominantly geographic, particularly in the 11 RPT patients where it was found in 7 out of 8 eyes with necrosis (Tables 46). These 7 patients with geographic necrosis included 3 who had complete tumor necrosis. Complete tumor necrosis was not found in the 27 eyes of patients who received preoperative RT or were treated with enucleation alone. In several cases, a single clear line of demarcation could be seen separating the necrotic zone from apparently viable tumor.
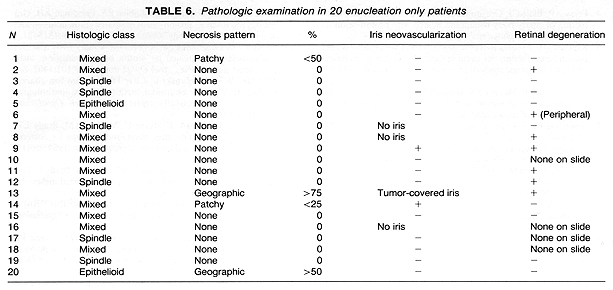
Neovascularization on the iris was present in 4 of 10 eyes examined following RPT. Sections of the remaining eye had insufficient iris for evaluation (Table 4). Of the 7 eyes enucleated following preoperative RT, none exhibited neovascularization on the iris. This included one eye where the tumor covered the iris, preventing evaluation of its surface (Table 5). It is of interest to note that 2 of 16 eyes with sufficient iris for evaluation in the enucleation alone group showed neovascularization (Table 6). These differences in the incidence of neovascularization on the iris by treatment group had no statistical significance.
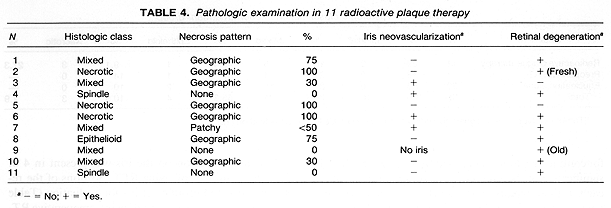
Retinal degeneration was found in 7 (70%) of the 10 eyes with retina present on the sections in the 11 eyes treated with RPT. This retinal degeneration included loss of ganglion cells and outer segments away from tumor in most cases, with others exhibiting tumor invasion, or peripheral cystic changes. Extensive retinal damage was present in 1 patient who had a treatment for retinal detachment prior to the diagnosis of choroidal melanoma. Retinal changes were found in two eyes of patients who received preoperative RT. In one of these two patients the changes consisted of peripheral cysts.
Of the 20 eyes in patients treated with enucleation alone, in 15 there was sufficient retina in the available sections to permit for the assessment of retinal degeneration away from the tumor (Table 6). In 7 of these 15 eyes there was evidence of global retinal damage. This included one eye with peripheral cystic changes and another one with a small retinal detachment. The remaining 5 patients had only a mild degree of ganglion cell loss.
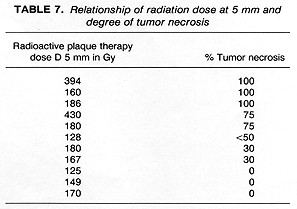
The influence of radiation dose on the presence, absence, or extent of tumor necrosis was difficult to determine in this study. There was, however, the suggestion of a correlation between the radiation dose and the amount of tumor necrosis expressed as >50% vs <50%. The eyes exhibiting a greater (>75%) degree of tumor necrosis were treated with higher radiation doses than those which had a lesser (<50%) degree of tumor necrosis or no necrosis (Table 7). The patients who received preoperative RT could not be evaluated for the relationship of dose to extent of tumor necrosis because they received the same dose of radiotherapy (20 Gy).
Scleral necrosis was present in 3 patients (27%) who had enucleation following RPT. There was no scleral necrosis found in the 27 eyes of patients who received preoperative radiotherapy or had enucleation alone.
The evaluation of treatment response in patients treated with RPT is a complex task. It includes detailed and repeated ophthalmic examinations to determine changes in tumor size, tumor characteristics, and changes in visual acuity. Histopathologic studies of enucleated eyes following RPT are of major importance. They can provide additional information on tumor response through the assessment of the extent of tumor necrosis. At the present time, the extent of tumor necrosis can not be reliably studied by any other method. An additional benefit of histopathologic examination is the ability to evaluate details of treatment complications.
In a study of 23 enucleated eyes following RPT with Cobalt-60, MacFaul and Morgan found signs of tumor necrosis in 6 specimens (26%) (2). Additionally, only 2 of these 6 patients had evidence of extensive necrosis (>50%). Postradiation changes, however, were present in nonmalignant ocular components in all 23 eyes examined. The reason for enucleation in 13 (56%) patients was progressive tumor or a lack of tumor regression. The remaining 10 patients (44%) developed severe treatment complications which required enucleation.
In contrast to the previous report (2), 72% of enucleated eyes in RPT patients in our study had evidence of tumor necrosis, including 45% who had extensive (>50%) tumor necrosis. The presence of tumor necrosis, however, should be interpreted cautiously, since, in our study, it was also found in 20% of patients who had enucleation alone (Table 3). Radiation dose may have an influence on the incidence and extent of tumor necrosis. The three eyes in our study that showed no evidence of tumor necrosis received the lowest radiation doses to the tumor. In contrast, 3 of the 6 patients who showed extensive (>50%) tumor necrosis, received a radiation dose >=250 Gy, calculated at 5 mm depth.
Several histopathologic studies have been reported following enucleation in patients treated for uveal melanoma with charged particle radiotherapy (3-5,14-16). Extensive postradiation changes were seen in the examined specimens. Tumor necrosis was a frequent finding. Its extent correlated well with the tumor size and pigmentation but not with the degree of tumor response assessed as a decrease in tumor dimensions (3).
Mitotic activity in the tumors examined by Crawford and Char (3) was low, which was interpreted as the evidence of radiation injury. Additional evidence for radiation injury was found in tumor capillaries (14,15). The changes found in tumor capillaries were felt to be compatible with those seen following radiation exposure. These included thickening of the vessel basement membrane, and perivascular fibrin deposits (14). Inflammatory response and lymphocytic infiltrates found in the examined tumors suggested the presence of an immune response (4,15).
The incidence of enucleation following RPT has been reported from a low of 6% to a high of over 17% (1,17-19). In a study of 59 enucleations following RPT given to 1,019 uveal melanoma patients (1,18) the reason for enucleation was persistent or recurrent tumor in 51% of these patients. The remaining patients had enucleation for treatment complications (17,18). In the present report, 55% of eyes were enucleated for treatment complications and 45% for persistent or recurrent tumor.
The existing literature provides strong evidence for the effectiveness of RPT in patients with uveal melanoma. This treatment has been evolving over the past 20 years with the resulting decrease in treatment complications (1,9). Episcleral radioactive plaque therapy, however, requires further optimization. The use of three-dimensional image reconstruction and three-dimensional radiation dosimetry could help to accomplish this goal (20,21). A routine application of this treatment optimization would be expected to decrease the incidence of treatment complications, resulting in an improvement in visual acuity. There is also a need to increase the incidence of local tumor control in RPT treated patients. Adjuvant episcleral hyperthermia is currently under study and its use may increase the effectiveness of episcleral radiotherapy by increasing the incidence of local tumor control (22,23).