Primary Malignant Melanoma of the Uvea: Radioactive Plaque Therapy and Other Treatment Modalities
Chapter 5 in Medical Radiology Radiotherapy of Intraocular and Orbital Tumors, ed. by W.E. Alberti and R.H. Sagerman, Copyright Springer-Verlag, Berlin, 1993. p. 31-44.
Zbigniew Petrovich, Melvin Astrahan, Gary Luxton, Jerry A. Shields, Carol L. Shields, and Luther W. Brady
ZBIGNIEW PETROVICH, M.D., Professor, Chairman; Department of Radiation Oncology, University of Southern California School of Medicine, Kenneth Norris Jr. Cancer Hospital and Research Institute, 1441 Eastlake Avenue, Los Angeles, CA 90033-0804, USA
MELVIN ASTRAHAN, Ph.D., Associate Professor, Director Radiation Physics; Department of Radiation Oncology, University of Southern California School of Medicine, Kenneth Norris Jr. Cancer Hospital and Research Institute, 1441 Eastlake Avenue, Los Angeles, CA 90033-0804, USA
GARY LUXTON, Ph.D., Associate Professor, Director Radiation Physics; Department of Radiation Oncology, Stanford University, Palo Alto, CA, USA
JERRY A. SHIELDS, M.D., Professor, Director, Oncology Service; CAROL L. SHIELDS, M.D., Assistant Professor; Wills Eye Hospital, Thomas Jefferson University, Ninth & Walnut Streets, Philadelphia, PA 19107, USA
LUTHER W. BRADY, M.D., Professor, Chairman, Department of Radiation Oncology and Nuclear Medicine, Halmemarm University, Broad & Vine Streets, Mail Stop 200, Philadelphia, PA 191021192, USA
CONTENTS
5.1 Introduction
Primary tumors of the eye are rare but important malignant lesions. In the United States, they represent less than 0.2% of all cancers (American Cancer Society 1990). Primary malignant melanoma of the uvea (MMU) is the most common primary tumor of the eye in adults. It constitutes 80% of primary eye tumors and about 6% of cutaneous melanomas (American Cancer Society 1990; CHALKELY 1980). This tumor is seen in older patients, with nearly equal frequency in males and females. MMU has been reported to be from 8 to 20 times more common among American whites than among blacks (CALLENDER et al. 1942; CAMPBELL WILDER and PAUL 1951; CHALKELY 1980).
5.2 Symptoms and Signs
The symptoms and signs of MMU are related to the site of origin and the size of the tumor. The most frequently observed symptoms, in order of their occurrence, are: blurred vision, partial subjective loss of vision, ocular pain, amaurosis, and photopsia (FITTERMAN and MCLEAN 1963). The same authors reported retinal detachment, presence of a mass, small nevi, intraocular bleeding, abnormal pupillary response, and glaucoma to be the most frequently seen signs.
5.3 Tumor Behavior
Excellent data on tumor behavior were reported from Denmark in a study of 302 patients diagnosed between 1943 and 1952. As expected, nearly all (97%) tumors originated in the choroid or choroid and ciliary body. The iris was involved in 3% (JENSEN 1982). The tumor is thought to grow by local expansion with progressive invasion of ocular and orbital structures. In a study reported from Vancouver, Canada, local tumor spread was seen in nearly one-third of patients (FITTERMAN and MCLEAN 1963). Sclera, vascular structures, and the orbit were most frequently involved by this local tumor spread, with optic nerve, iris, and lymphatic involvement seen less frequently (FITTERMAN and MCLEAN 1963; WRIGHT 1949). Vitreous involvement is seen in patients with large and necrotic tumors (SPENCER 1975). The presence of melanoma cells in the vitreous results in a very poor prognosis (SHAMMAS and BLODI 1977a).
Local recurrence in the orbit is surprisingly uncommon. Its incidence has been reported at a low of 2.5%. A sharp increase to 12% is seen in patients with extrascleral tumor extension (JENSEN 1982). The incidence of orbital recurrence was nearly twice as high following enucleation in patients with extrascleral extension (AFFELDT et al. 1980). Orbital recurrence is usually seen within 2 years of enucleation (JENSEN 1982) and is controlled in only 40%. The presence of local recurrence correlated well with the presence of metastasis and death due to metastatic disease (AFFELDT et al. 1980; SHAMMAS and BLODI 1977a,b).
The primary mode of death in patients with MMU is metastatic disease, seen in 51% of patients (JENSEN 1982). Metastases occur early, before 2 years, in the majority of patients (JENSEN 1982; LEAN et al. 1990). Occasionally, however, a patient may develop clinical evidence of metastatic disease more than 20 years after diagnosis (JENSEN 1982). The liver is the most common site of metastatic disease in patients with MMU, in contrast to those with malignant melanoma of other primary sites, where liver involvement is seen in 20% of patients (WRIGHT 1949; CHAR 1978; RAJPAL et al. 1983; JENSEN 1982; DONOSO et al. 1986; EINHORN et al. 1974a,b).
5.4 Histology
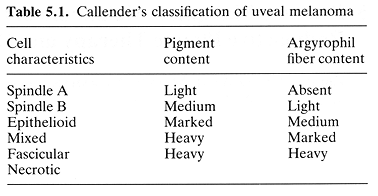
Malignant melanoma of the uvea was classified into several groups (CALLENDER et al. 1942). These groups were based on cell characteristics, pigment content, presence of tumor necrosis, and content of argyrophilic fibers (Table 5.1). These categories were of importance because they predicted the outcome of treatment. The best survival was seen in patients with spindle A and B types, those with heavy content of argyrophilic fibers, while those with light or absent argyrophilic fibers had a poor prognosis (CALLENDER et al. 1942). Forty years later, modifications proposed by McLEAN et al. (1983) allowed for even better correlation between histological characteristics and treatment result.
5.5 Pretreatment Evaluation
The goals of pretreatment assessment include establishing the diagnosis of melanoma, defining the tumor's anatomical extent, location, and dimensions, and identifying its important characteristics such as degree of pigmentation and echographic properties. Histological confirmation of the clinical diagnosis is usually established only following enucleation. The pretreatment diagnosis of MMU can be established noninvasively with a great degree of accuracy in most patients (SHIELDS and SHIELDS 1992). A detailed general and ophthalmic history is essential. General physical examination is directed toward discovering clinical evidence of metastatic disease. This is followed by a detailed ophthalmic examination which includes general eye examination, visual acuity, fundus photography, and biomicroscopy. Ultrasound examination, using A and B modes, is performed in all patients. Although nonspecific, this examination plays a critical role in helping to establish the diagnosis as well as to obtain accurate tumor dimensions. Due to its importance in the diagnostic process, it should be performed by an ophthalmic ultrasound specialist rather than by a general diagnostic radiologist or general ultrasound specialist. Computed axial tomography (CAT) and magnetic resonance imaging (MRI) studies are not in routine use at this time. Their value in MMU is yet to be established (GOMORI et al. 1986). Some centers perform fluorescein angiography. The radioactive phosphorus uptake test is of little value at the present time.
Other studies include chest radiography, complete blood count, serum alkaline phosphatase, and serum lactic acid dehydrogenase. These basic studies, with a judicial use of other tests and intelligent interpretation of information obtained from the history and physical examination, are usually sufficient to establish a diagnosis of MMU and the presence or absence of metastatic disease (EINHORN et al. 1974a; LEAN et al. 1990; SHIELDS and SHIELDS 1992). It is imperative to histologically confirm suspected metastases with an image-directed needle biopsy.
In the 1960s, diagnostic accuracy in MMU was about 80% (FERRY 1964; SHIELDS and ZIMMERMAN 1973). Diagnostic accuracy has increased over the years and an incorrect diagnosis is now unusual. In a report from the Wills Eye Hospital on 1398 consecutive enucleations, the diagnostic accuracy was nearly 97%; accuracy increased to 98% for the last 103 patients (SHIELDS and McDONALD 1974). Recently, the Collaborative Ocular Melanoma Study (COMS) reported histological
confirmation of the clinical diagnosis in 411 of the 413 eyes examined (COMS 1990).
Diagnostic problems may be seen particularly in patients with small (<3 mm in height) tumors. In such cases, it is advisable to defer therapy until evidence of tumor progression is noted. The importance of this approach was recently reemphasized in a report on the management of 3000 patients with MMU where 411 (14%) patients belonged to the observation group (SHIELDS and SHIELDS 1992). During follow-up examination, occasionally retinal detachment, the presence of benign tumors, or early metastatic tumor to the eye will become apparent (FERRY 1964). As a final step in pretreatment evaluation, accurate staging is required.

Two staging systems are currently being used by ophthalmic oncologists. The first system is the one recommended by the American Joint Committee on Cancer 1988 (Table 5.2). The second one is recommended by the COMS (EARLE et al. 1987) (Table 5.3).

5.6 Treatment
Surgical treatment and radioactive plaque therapy (RPT) are widely used curative modalities in the management of patients with MMU. Photoradiation, photocoagulation, cryosurgery, and hyperthermia are either of historical interest or are in phase I clinical trials.
5.6.1 Surgery
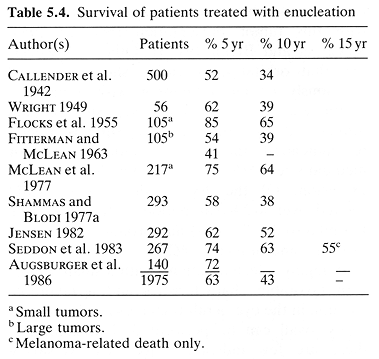
Surgical treatment has been utilized in the management of ocular melanoma for more than 100 years. The most frequent procedure is enucleation, used in the treatment of 35% of patients (SHIELDS and SHIELDS 1992). There is virtually no surgical mortality, low morbidity, and good longterm survival (SHIELDS and SHIELDS 1992). In a compilation of nine reported series consisting of 2024 enucleations, the 5- and 10-year survivals rates were 63% and 43%, respectively (Table 5.4). In a series of 292 enucleations with 99% long-term follow-up, the 25-year survival rate was 40% (JENSEN 1982).
Multiple important prognostic factors have been identified. They include the features identified microscopically as well as clinically (THOMAS et al. 1979; SHAMMAS and BLODI 1977a; SEDDON et al. 1983; MCLEAN et al. 1977; SHIELDS and SHIELDS 1992). Tumor volume is one of the most important prognostic factors. In a group of 292 patients with a 25-year period of observation, death due to metastatic disease was seen in 44% of patients with tumors <=100mm³ and in 63% of those with larger (> 100 mm³) tumors (P < 0.001) (JENSEN 1982).
Optic nerve invasion and juxtapapillary tumor location are widely considered to be adverse prognostic factors but are overshadowed by other factors such as poor histology, vitreous invasion, or inadequate surgical margins (SPENCER 1975; WEINHAUS et al. 1985).
Patients with orbital tumor extension experience an almost tenfold increase in the incidence of postenucleation orbital recurrence (SHAMMAS and BLODI 1977b). Orbital exenteration has been recommended by some investigators for patients with this tumor presentation (NAQUIN 1954; SHAMMAS and BLODI 1977b). Others, however, feel that orbital exenteration should be used judiciously for selected patients with extrascleral tumor extension (JENSEN 1982; SHIELDS and SHIELDS 1992). SHIELDS and SHIELDS (1992) recommended the use of radiotherapy for medium-sized MMU with minimal extrascleral extension. Of the 3000 patients with MMU treated over a 20-year period at the Wills Eye Hospital, only 125 (4%) had orbital exenteration (SHIELDS and SHIELDS 1992).
In highly selected patients with a small tumor, with favorable characteristics and a favorable location in the eye, a full-thickness local resection of eye wall can be performed. Unfortunately, there are few indications for this eyesaving surgical procedure (PEYMAN and APPLE 1974; SHIELDS and SHIELDS 1992).
Metastatic disease has been reported as a cause of death in 51% of enucleated MMU patients (JENSEN 1982). There is no effective treatment available for metastatic ocular melanoma (RAJPAL et al. 1983; CHAR 1978). The median survival of patients with metastatic liver disease is 8 weeks (RAJPAL et al. 1983). To minimize the possibility of tumor cell dissemination during surgery (BURNS et al. 1962), a no-touch technique has been advocated (FRAUNFELDER et al. 1977). The effectiveness of this technique has been difficult to assess.
In the late 1970s, it was hypothesized that MMU had a low metastatic potential, particularly in its early stages (ZIMMERMAN et al. 1978; ZIMMERMAN and MCLEAN 1979). These authors also suggested that enucleation itself helped to disseminate tumor cells and promote metastatic disease. This challenge to enucleation resulted in a call to establish a moratorium on this procedure. Subsequent work reported by SEIGEL et al. (1979) demonstrated no increase in the incidence of metastatic disease in patients with MMU who were treated with enucleation.
5.6.2 External Beam Radiotherapy
5.6.2.1 Adjuvant External Beam Radiotherapy
An interesting study of 110 patients with MMU treated with 50Gy of postoperative orbital irradiation was reported by SOBANSKI et al. (1972). The authors concluded that the use of postoperative irradiation was of major benefit to their patients. It reduced the 5-year mortality from 69% to 19%, the 10-year mortality from 85% to 41%, and the 15 year mortality from 89% to 58% compared with those who were treated with enucleation only. However, this study was inconclusive since it did not compare the treatment arms in a prospective randomized trial.
A more recent report of a phase I/III study evaluated the role of preoperative orbital irradiation in 41 MMU patients (CHAR et al. 1988). this group was compared with 31 matched patients treated previously with enucleation alone. There was no benefit from preoperative irradiation (20 Gy in 5 equal daily fractions) in terms of tumor-related survival. This study is also considered inconclusive for the same reason as the aforementioned study. This led the COMS to initiate a prospective randomized trial comparing enucleation alone with preenucleation external beam radiotherapy. The randomized MMU patients were required to have large tumors (group III). When completed, this study may provide conclusive information on the value of adjuvant radiotherapy preceding enucleation.
5.6.2.2 External Beam Photon Radiotherapy
Conventional photon beam irradiation was used in the past as primary treatment to control distressing symptoms and signs of locally advanced MMU (LEDERMAN 1956). More recently an attempt was made to treat selected posterior MMU with a modified Co-60 teletherapy unit. The radiation dosimetry, as it was presented, appeared interesting (CHENERY et al. 1977). It was not clear, however, whether this system was used for actual treatment of MMU patients. RAND et al. (1989) presented an interesting proposal to utilize Leksel's stereotactic radiosurgery system for treatment of melanomas of the eye. Evaluation of this system has to await accumulation of clinical data.
5.6.2.3 External Beam Particle Radiotherapy
External beam radiotherapy with charged particles has been extensively and effectively used in the management of patients with MMU. Helium ion beam has been used at the University of California Lawrence Berkeley Laboratory since 1977 (CHAR et al. 1980; CHAR and CASTRO 1982; SAUNDERS et al. 1985) (see also Chap. 6).
Treatment efficacy with the use of helium ions in 312 MMU patients was compared with that in 157 matched MMU patients treated with I-125 episcleral plaque radiotherapy. Most of the treated patients had medium-sized or large tumors. The 5-year tumor-related mortality for the 469 patients was 23% and there was no significant difference in survival, incidence of enucleation, incidence of metastatic disease, or treatment complications between the helium ion and the I-125 plaque treated patients (CHAR et al. 1989).
Proton beam irradiation for patients with MMU has been under study in Boston since 1974 (GRAGOUDAS et al. 1980, 1982; AUSTIN-SEYMOUR et al. 1985). A total of 615 patients were treated, with tumor regression noted in 94%. Additionally, two-thirds of the patients had visual acuity of at least 20/200 (AUSTIN-SEYMOUR et al. 1985) (see Chap. 6). Both particle beams remain a viable alternative to enucleation and episcleral RPT.
5.6.3 Radioactive Plaque Therapy
Radioactive plaque therapy is a widely used treatment mode that provides an alternative to surgical therapy (BRADY et al. 1982; AUGSBURGER et al. 1986; PACKER and ROTMAN 1980). RPT began more than 50 years ago with the use of Radon seeds and, subsequently, with Co-60 applicators (STALLARD 1966).
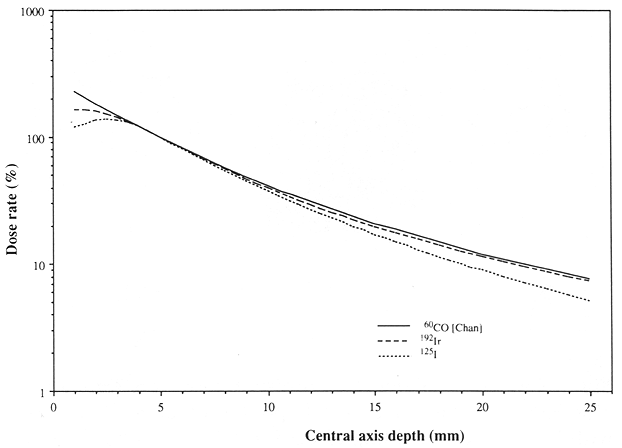
Fig. 5.1. Comparison of relative central axis dose rates for 12-seed, 15-mm diameter plaques of Ir-192 and I-125 with that from a Stallard Co-60 plaque. All plaque dose rates are normalized to 100% at the point on the central axis 5 mm from the plaque surface. The higher dose rate found for the Co-60 plaque at distances of 1-2mm from the plaque surface is an artifact due to the presence of radioactive material on the central axis in the Stallard plaque. The seed plaques were designed to position all seeds off the central axis, and the off-axis dose rate adjacent to a seed is comparable to the Co-60 central axis dose rate. Central axis dose rates in the 3- to 8-mm region are similar for all three plaques. The dose rate for the I-125 plaque is 25 % - 35 % less than that for Co-60 at the eye surface remote from the plaque, but for both types the dose rate is less than 10% of the dose rate at the reference depth of 5 mm. [Co-60 data are for the Stallard CKA-4 plaque, and are adapted from CHAN et al. (1972); the other data are taken from LUXTON et al. (1988a).]
In the 1970s RPT was introduced to the United States with radioactive I-125 by PACKER and ROTMAN and with Co-60 by BRADY and SHIELDS (PACKER and ROTMAN 1980; BRADY et al. 1982; SHIELDS et al. 1982). Subsequent work resulted in optimization of the treatment technique, with routine use of multiple radioactive isotopes such as Ir-192, I-125, Ru-106/Rh-106, Co-60, and, more recently, Pd-103 (LOMMATZSCH 1983; SCARBOROUGH et al. 1988; FINGER et al. 1991). At the present time the most widely used isotope for RPT is I-125. This is primarily because of its superior dosimetry and radiation protection considerations as compared with Co-60 or even Ir-192 (Fig. 5.1). The use of Rn-222 and Au-198 RPT is largely of historical interest due to less than optimal dosimetry and radiation safety concerns (NEWMAN et al. 1970; DAVIDORF et al. 1987; CHENERY et al. 1983).
5.6.3.1 Dosimetry
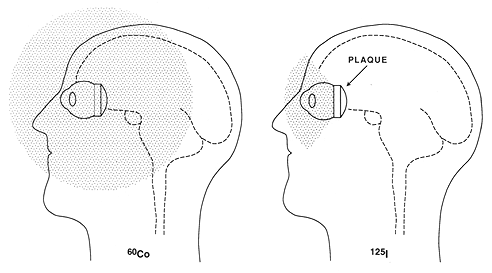
Fig. 5.2. A schematic representation of shielding efficiency with the use of a I-125 episcleral plaque compared with lack of this shielding effect for a Co-60 plaque. Note a far greater amount of intraocular and extraocular tissue being exposed to high doses of radiation with the Co-60 plaque
Much work has been done in the past 25 years to understand the subtleties of RPT dosimetry. Early efforts focused on dosimetry of the standard Stallard Co-60 plaques (STALLARD 1961, 1966; MAGNUS 1967; CASEBOW 1971; CHAN et al. 1972; BEDDOE 1975). Dosimetry of Au-198 seeds sutured directly to the sclera was discussed by CHENERY et al. (1983). Dosimetry of gold plaques containing Ir-192 was studied by LUXTON et al. (1988a). The authors noted no dosimetric contraindications to the use of Ir-192 or I-125 in place of Co-60 for choroidal melanomas of up to 10 mm in elevation. In fact, the relative central axis dose rates over the distance of 3-10mm from the plaque surface was quite similar for Co-60, Ir-192, and I-125 (Fig. 5.1). Currently, I-125 is preferred because its lower energy permits easy shielding, leading to better radiation protection for personnel and better protection of ocular and extraocular normal tissues than can be accomplished with the use of plaques containing Co-60 or Ir-192 (Fig. 5.2). Palladium 103 seeds recently became available for use in RPT (SCARBOROUGH et al. 1988; FINGER et al. 1991). The average energy of 103 Pd radiation is lower (21keV) than that of I-125 (28 keV) but this difference is not important clinically. These isotopes can easily be shielded with >99% direct attenuation obtained using thin (0.2mm) gold or lead foil. This ease of attenuation is of major clinical importance since it permits a more optimal radiation dose distribution through the volume of interest (Fig. 5.3). The shorter half-life (17 days) of Pd-103 makes it less convenient to reuse sources (and more expensive) than with I-125 (half-life: 60 days).
The complexity of radiation dose distribution from individual I-125 seeds was noted by investigators several years ago (KRISHNASWAMY 1978; LING et al. 1983, 1985). The importance of this complexity in dose distribution for episcleral plaque therapy was studied extensively (WEAVER 1986; LuXTON et al. 1988b). The outcome of these studies was a 10%-18% reduction in the estimated dose from calibrated I-125 seeds (WEAVER et al. 1989; LuXTON et al. 1990; CHIUTSAo et al. 1990; NATH et al. 1990).
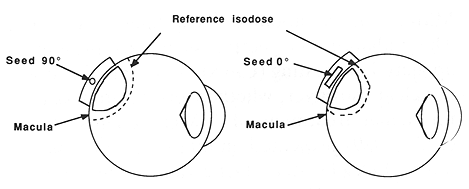
Fig. 5.3. A schematic representation of the importance of controlling the I-125 seed orientation in an episcieral plaque and its influence on the radiation dose to the uninvolved macula. On the left side the I-125 seed is at a 90° angle and the macula is within the high dose volume. On the right side, changing the orientation of the seed to a 0° angle leaves the macula outside the reference high dose isodose line. This change may result in a substantial (>30%) dose reduction to the macula
The complexity of I-125 seed dosimetry was applied to optimize treatment planning (ASTRAHAN et al. 1990). The authors were able to substantially reduce radiation dose to the important uninvolved structures of the eye, such as the macula, while leaving the tumor dose unchanged. The anisotropy of the dose distribution from I-125 seeds enables the physical orientation of individual seeds to play a major role in the resulting treatment plan (Fig. 5.3). Utilization of this property of I-125 seeds in clinical practice requires a 3-D treatment planning system. The system employed at the University of Southern California (USC) takes into account the location of important structures in relation to the plaque seeds, as well as processing a 2-D calculational model capability for radiation dose from individual seeds (ASTRAHAN et al. 1990a,b; LING et al. 1989). Additionally, it is useful to include the dose modifying and shielding properties of the plaque itself (ASTRAHAN et al. 1990a,b).
The implementation of optimized dose planning with individual I-125 seeds in RPT can substantially reduce the radiation dose to adjacent but uninvolved structures such as the macula or optic nerve. This dose reduction is in the range of 30%-50% when compared with an unselected seed orientation and the use of equal intensity seeds in a given episcleral plaque. However, dose optimization can be fully successful only if the surgical placement is accurately preplanned and executed, based on guidance from a full 3-D dose calculation. The model of the eye should be based on patient-specific data such as those obtained from CAT.
The use of Sr-90/Y-90 and Ru-106/Rh-106 radioactive plaques allows for more rapid dose fall off than is seen with the use of Co-60, Ir-192, or I-125 plaques. This difference in dose rate fall off may permit more selective use of radioactive plaques containing different isotopes, thus further optimizing dose distribution in a given clinical situation (SINCLAIR and TROTT 1956). This selective use of radioactive isotopes in the treatment of tumors of different size has been applied clinically (BRADY et al. 1988; SEALY et al. 1976; LOMMATZSCH 1983, 1986).
5.6.3.2 Technique
The basic technique consists of intraoperative localization of choroidal melanoma and suturing of a radioactive plaque to the sclera overlying the tumor. Several variations of this technique are in clinical use.
The technique used at the USC School of Medicine is described here, in detail. Since 1986, I-125 has been used exclusively as the radioactive isotope. This decision was based on radiation safety considerations and more optimal dosimetry with the use of I-125 seeds (LUXTON et al. 1988b; ASTRAHAN et al. 1990b).

Fig. 5.4. Examples of episcleral plaques as used at USC. Note the notch permitting effective dose delivery to tumors adjacent to the optic nerve
Multiple gold-shelled plaques are available to meet virually any clinical need. Examples of USC and COMS plaques are shown in Figs. 5.4 and 5.5. The USC plaques permit better dose optimization and allow the treatment of lesions adjacent to the optic nerve. The COMS plaques have a somewhat lower scleral dose. However, consideration has to be given to the attenuating property of Silastic inserts in the COMS plaques, which may result in a 10% dose reduction (KAROLIS et al. 1990).
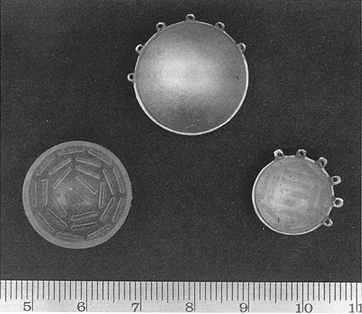
Fig. 5.5. Episcleral plaques used by the COMS
Plaque placement and removal are performed in the operating room as outpatient procedures. Intravenous sedation is given in addition to retrobulbar and eyelid blocks with a local anesthetic. This procedure gives excellent local anesthesia and akinesia. The conjunctiva is opened at the limbus, centered in the location of the tumor, and Tenon's capsule is dissected off the sclera. The rectus muscles are isolated and traction with 00 silk sutures is applied. Detailed examination of the affected eye is performed using transillumination. Marks are placed on the sclera to outline the tumor base and a nonradioactive plaque, simulating the one to be used for treatment, is sutured to the sclera. The position of the 'cold' plaque is verified directly and with the use of transillumination. Once a satisfactory position is ascertained, a radioactive plaque is quickly placed in the treatment position and its position verified. The conjunctiva is closed with plain catgut sutures.
Prior to completion of procedure, a subTenon's injection of gentamycin (20mg) and dexamethasone (40mg) is given. A special lead shield is placed over an eye patch, dramatically reducing transmission of radiation. The patient is discharged home and returns for removal of the implant.
This procedure has been very well tolerated in more than 130 patients. The details of plaque dosimetry have been published (LUXTON et al. 1988a,b, 1990; PETROVICH et al. 1990b).
5.6.3.3 Results
In a recent report from the Wills Eye Hospital, based on that Medical center's 20-year experience with 3000 MMU patients, RPT was the most frequently (51%) used treatment modality (SHIELDS and SHIELDS 1992). Treatment results have been excellent. In a study of 100 patients treated with Co-60 plaques, the 5-year survival rate was 86% as compared with 50% in 300 patients treated with enucleation in the same institution (MAcFAUL and MORGAN 1977). AUGSBURGER et al. (1986) reported a nonrandomized comparison of 169 patients treated with enucleation and 103 similar patients treated with Co-60 plaques. There was a significant (P < 0.05) survival advantage in the 103 Co-60 treated patients and a lower incidence of death due to melanoma. An additional advantage for conservatively treated patients was preservation of eye function in most of these patients. Similar excellent results have been published over the years from the same center (CRUESS et al. 1984; BRADY et al. 1988; KARLSSON et al. 1989; MARKOE et al. 1985; SHIELDS and SHIELDS 1992). Treatment of 29 RPT patients, using I-125, resulted in an 83% survival rate with a mean follow-up of 38 months and in a study of 28 patients 89% survival rate with a mean follow-up of 39 months (PACKER 1987; PACKER et al. 1984) (Table 5.5).
A large series of patients (309) treated with plaques containing a Ru-106/Rh-106 beta emitter, with a mean follow-up of more than 6 years, showed 84% 5-year and 66% 10-year survival rates (LOMMATZSCH 1986).
In a compilation of six series consisting of 794 RPT patients, the 5-year survival rate ranged from a low of 70% to a high of 91% (mean 82%). This compares with 2024 enucleation-treated patients in nine series in which the 5 year survival rate ranged from 41% to 85% (mean 63%).
Despite these convincing results favoring RPT patients, measured in terms of tumor response rate, 5-year survival rate, and the incidence of melanoma-specific death, management of MMU remains unsettled. This is primarily due to the absence of prospective, randomized trials. The COMS was formed in an attempt to answer these important management questions (EARLE et al. 1987; COMS 1990).
Three studies are being conducted. Study I is a randomized comparison of enucleation versus RPT in patients with tumors >=3.1 <=8mm in height and/or <=16mm in basal diameter. Study II is a prospective randomized comparison of enucleation alone versus enucleation with adjuvant preoperative external beam radiotherapy in patients with large group III tumors (>8 mm in height and/or >16mm in basal diameter). The third study was designed to prospectively assess he need for treatment in patients with small or group I tumors (<=3 mm in height). The results of these studies are eagerly awaited.
5.6.3.4 Complications
Ocular radiation toxicity data are available from head and neck cancer patients who received irradiation to part or all of an eye (BRADY et al. 1989; CHAN and SHUKOVSKY 1976; HAM 1953; MacFAUL and BEDFORD 1970; NAKISSA et al, 1983; PARSONS et al. 1983). Radiation injuries of significance, such as those affecting the optic nerve or retina, predictably occur among patients who received >60Gy to the eye (PARSONS et al. 1983; NAKISSA et al. 1983). In spite of this high dose, two-thirds of the patients receiving >60 Gy to the eye showed no clinical evidence of major injury (CHAN and SHUKOVSKY 1976).
The above data, however, are not directly applicable to the treatment of MMU patients with RPT, where radiation dose schedules and the volume of the eye treated are quite different.
Acute complications from RPT are of little clinical significance. The incidence of late radiation injuries varies in the reported series and depends on the tumor's location in the eye, the radioactive isotope used, the radiation dose both at the tumor apex and at the sclera, pretreatment function of the eye, and size. the overall incidence of late complications is up to 40% (STALLARD 1966; PACKER et al. 1984; LOMMATZSCH 1986; BRADY et al. 1989; MacFAUL and BEDFORD 1970; LEAN et al. 1990). A commonly seen complication is a perimacular exudate, which undergoes a slow resolution (STALLARD 1966). Retinal and vitreous hemorrhages, and vascular changes leading to retinopathy, are also common (STALLARD 1966; MacFAUL and BEDFORD 1970). The presence of radiation cataract in the irradiated eye, although important, is relatively easy to correct with a simple surgical procedure (COGAN et al. 1952; HAM 1953; MERRIAM and FOCHT 1957). A serious late effect of RPT is scleral necrosis, which may require enucleation even in the absence of tumor progression (CAPPIN 1973; MacFAUL and MORGAN 1977; PETROVICH et al. 1990a,b).
Post-RPT enucleation is an important event. There are an equal number of patients who require enucleation for treatment complications and those who have persistent or progressive recurrent disease (MacFAUL and MORGAN 1977). The total incidence of post-RPT enucleation from all causes is about 20% (MacFAUL and MORGAN 1977; LOMMATZSCH 1986; LEAN et al. 1990). In one study following the introduction of treatment optimization, the incidence of enucleation has decreased from 46% to 2% (PETROVICH et al. 1991).
Visual acuity is an important endpoint in RPT patients. A recent report on 100 Co-60-treated patients showed a gradual decrease in visual acuity which amounted to a 10% annual reduction (BRADY et al. 1988). This must be considered in the context of the loss of sight after enucleation.
A recent report on the treatment of 85 MMU patients showed a sharp decrease in RPT complications following the introduction of an extensive treatment optimization program (PETROVICH et al. 1991). This optimization program included: a change of radioactive isotope from Ir-192 to I-125, more selective radiation dose distribution, routine use of treatment preplanning with 3-D dose distribution, and the use of radioactive I-125 seeds of different activities in the same plaque.
A review of the available data on RPT complications shows good treatment tolerance in most patients, with an acceptable incidence of late complications. The incidence of tumor eradication is high, with preservation of useful vision in the majority of patients. This information tends to bias most patients and some clinicians to favor this conservative therapy over enucleation.
5.6.3.5 Future Directions
The fundamental objective of RPT is to control malignancy while maintaining useful vision. Present RPT techniques result in a high incidence of tumor control for intermediate and small lesions (<=8mm in height). Tumor control for large lesions (>8mm in height and/or >16mm basal diameter) is not optimal. Additionally, there is a higher incidence of late complications resulting in impaired vision with large lesions (MacFAUL and MORGAN 1977; PETROVICH et al. 1990b). It is likely that radiation dose reduction to the uninvolved part of the eye will reduce the incidence of late complications while maintaining a high incidence of tumor control for smaller tumors and perhaps increasing it for larger lesions. To accomplish this goal, one may choose to use radioactive plaques containing I-125, Pd-103, or a beta emitter, such as Ru-106/Rh-106 rather than Co-60 or Ir-192. The use of I-125 allowed the USC group to perform treatment on an outpatient basis. The low keV radioactive isotopes will also permit use of the collimating effect of the gold plaque by deepening the grooves to receive the seeds. Ultimately, the use of this seed collimation is expected to result in a reduction of the steep dose gradient between tumor apex and the sclera (PETROVICH et al. 1991).
It is expected that major benefit in terms of treatment optimization and lower incidence of late complications will be obtained by use of 3-D imaging combined with 3-D radiation dosimetry. Such a 3-D system (Plaque Simulator) was developed at USC and is currently undergoing testing in multiple medical centers. This system permits precise pretreatment planning and modifications of the plan at short notice, such as in the case of new intraoperative findings. Precision of the treatment plan is assured by the direct use of real-time imaging with CAT or MRI (ASTRAHAN et al. 1990a,b).
A major increase in the incidence of tumor control is expected with the use of adjuvant hyperthermia (HT). Several ophthalmic HT systems have been developed and are currently undergoing phase I clinical trials. A microwave heating technique designed for ocular malignancy has been found particularly useful in locally advanced retinoblastorna (LAGENDIJK 1982). A combination of proton irradiation and ultrasound-induced hyperthermia was tested in an animal model (RIEDEL et al. 1985). Hyperthermic temperatures were achievable with potentiation of the antitumor effect of proton radiotherapy. The authors concluded that adjuvant HT has the potential to improve tumor control and lower the incidence of late complications.
A different ultrasound-generated HT system was developed by a group at Cornell University (COLEMAN et al. 1986). In a small phase I study, good tumor regression was noted with conventional Co-60 RPT. Lower doses of radiation apparently showed tumor regression expected with the use of much higher (30%-50%) radiation doses. This was viewed by the authors as probable evidence of a synergistic effect of the RT-HT combination and raised the hope of lowering the incidence of late complications of radiotherapy.
Simultaneous thermoradiotherapy with the use of microwaves and I-125 plaques was decribed by FINGER et al. (1985,1989). Results of a phase I study with 18 MMU patients showed good tumor regression in all and no evidence of early or late complications which could be attributed to the use of adjuvant HT.
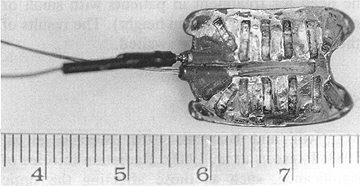
Fig. 5.6. A USC episcleral plaque for thermoradiotherapy
A low frequency (500kHz) HT system designed for simultaneous application with a I-125 plaque was reported by ASTRAHAN et al. (1987) (Fig. 5.6). This system is capable of delivering hyperthermic temperatures to the apical part of a choroidal melanoma. In an animal study, a graded effect was noted with increasing temperature and this effect was confined to the heated area. No significant acute toxicity was observed with temperatures of up to 45°C applied for 45min. A higher temperature caused serious retinal injury with an extinguished electroretinogram. A diffuse photoreceptor injury was seen both inside and outside the treatment field. These findings were confirmed histologically and with ultrastructure studies (LIGGETT et al. 1990). In a phase I clinical trial of 21 patients with locally advanced and/or recurrent tumors, strong evidence of therapeutic activity was seen in these thermoradiotherapy-treated patients (PETROVICH et al. 1992).
Based on the above-mentioned thermoradiotherapy studies, phase II clinical trials are being started. It is very likely that the use of ocular thermoradiotherapy will result in a substantial improvement in local tumor control. The use of HT with lower than generally administered doses of radiotherapy is also being investigated. These lower doses of irradiation will be expected to reduce the incidence of late radiation injury. At the same time, the level of sophistication of the available ocular HT systems is improving rapidly.
5.6.4 Other Treatment Modalities
Cryosurgical treatment was used in the past in an attempt to control choroidal melanoma and other ocular tumors. The results of this treatment were equivocal. Cryosurgery was felt to be of palliative or adjuvant value in selected patients (LINCOFF et al. 1967). At the present time, cryosurgery is used infrequently, prior to enucleation for large MMU.
Photocoagulation or diathermy is largely of historical interest and is not widely utilized for the treatment of patients with MMU (BONIUK and
GIRARD 1965; VOGEL 1972). An interesting development during the past decade is the use of photoradiation therapy following the administration of hematoporphyrin derivatives. Studies with photodynamic therapy preceded by the
administration of hematoporphyrin derivatives (HPD) have been conducted at USC (GOMER et al. 1983, 1987). The results suggest HPD photodynamic therapy to be a feasible and safe therapeutic approach. Much more work, however, needs to be done before this promising treatment can be tested in clinical trials.
5.7 Summary
There is overwhelming evidence that malignant melanoma of the uveal tract can be treated safely with radioactive plaques with long-term survival rates equal to those of enucleation. Preservation of eye function is expected in the majority of RPT-treated patients. Application of low energy isotopes, collimation of individual seeds, and routine use of 3-D imaging and 3-D dosimetry should help to further optimize episcleral plaque therapy. New developments in ocular thermoradiotherapy allow for some degree of optimism and hope of better tumor control in large choroidal melanomas and a reduction in the incidence of serious late radiation complications.
References
- Affeldt JC, Minckler DS, Azen SP et al. (1980) Prognosis in uveal melanoma with extrascleral extension, Arch Ophthalmol 98:1975-1979
- American Cancer Society (1990) Cancer Statistics 1990. CA 4:9-26
- American Joint Committee on Cancer (1988) Melanoma of the uvea. In: Behrs OH (ed) Manual for staging of cancer. JB Lippincott, Philadelphia, pp231-233
- Astrahan MA, Liggett PE, Luxton G et al. (1987) A 500kHz localized current field hyperthermia system for use with ophthalmic plaque radiotherapy. Int J Hyperthermia 3:423-432
- Astrahan MA, Luxton G, Jozsef G et al. (1990a) An interactive treatment planning system for ophthalmic plaque brachytherapy. Int J Radiat Oncol Biol Phys 18:679-687
- Astrahan MA, Luxton G, Jozsef G et al. (1990b) Optimization of I-125 ophthalmic plaque brachytherapy. Med Phys 17:1053-1057
- Augsburger JJ, Gamel JW, Sardi VF et al. (1986) Enucleation vs. cobalt plaque radiotherapy for malignant melanomas of the choroid and ciliary body. Arch Ophthalmol 104:655-661
- Austin-Seymour M, Munzenrider JE, Goitein M et al. (1985) Progress in Low-LET heavy particle therapy: intracranial and paracranial tumors and uveal melanomas. Radiat Res 104:S219-S226
- Beddoe AH (1975) Isoexposure curves for Co-60 ophthalmic applicators. Aust Radiol 19:145-151
- Boniuk M, Girard U (1965) Malignant melanoma of the choroid treated with photocoagulation, transscleral diathermy and implanted radon seeds. Am J Ophthalmol 59:212-216
- Brady LW, Shields JA, Augsburger JJ et al. (1982) Malignant intraocular tumors. Cancer 49:578-585
- Brady LW, Markoe AM, Amendola BE et al. (1988) The treatment of primary intraocular malignancy. Int J Radiat Oncol Biol Phys 15:1355-1361
- Brady LW, Shields J, Augsburger J et al. (1989) Complications from radiation therapy to the eye. Front Radiat Ther Oncol 23:238-250
- Burns RP, Fraunfelderr FT, Klass AM (1962) A laboratory evaluation of enucleation in the treatment of intraocular malignant melanoma. Arch Ophthalmol 67:490-500
- Callender GR, Wilder HC, Ash JE (1942) Five hundred melanomas of the choroid and ciliary body followed five years or longer. Am J Ophthalmol 25:962-967
- Campbell Wilder H, Paul EV (1951) Malignant melanoma of the choroid and ciliary body: a study of 2535 cases. Milit Surg 109:370-378
- Cappin JM (1973) Radiation scleral necrosis simulating early scleromalacia perforans. Br J Ophthalmol 57:425-428
- Casebow MP (1971) The calculation and measurement of exposure distributions from Co-60 ophthalmic applicators. Br J Radiol 44:618-624
- Chalkely T (1980) Ocular Melanoma Task Force report. Am J Ophthalmol 90:728-733
- Chan B, Rotman M, Randall GJ (1972) Computerized dosimetry of Co-60 ophthalmic applicators. Radiology 103:705-707
- Chan RC, Shukovsky LJ (1976) Effects of irradiation on the eye. Radiology 120:673-675
- Char DH (1978) Metastatic choroid melanoma. Am J Ophthalmol 86:76-80
- Char DH, Castro JR (1982) Helium ion therapy for choroidal melanoma. Arch Ophthalmol 100:935-938
- Char DH, Castro JR, Quivey JM et al. (1980) Helium ion charged particle therapy for choroidal melanoma. Ophthalmology 87:565-570
- Char DH, Phillips TL, Andejewski Y et al. (1988) Failure of pre-enucleation radiation to decrease uveal melanoma mortality. Am J Ophthalmol 106:21-26
- Char DH, Castro JR, Quivey JM et al. (1989) Uveal melanoma radiation I-125 brachytherapy versus helium ion irradiation. Ophthalmology 96:1708-1715
- Chenery SAG, Galbraith DM, Leung PMK (1977) Application of small Co-60 beams in the treatment of malignant melanoma at the optic disc. Int J Radiat Oncol Biol Phys 2:1021-1026
- Chenery SAG, Japp N, Fitzpatrick PJ (1983) Dosimetry of radioactive gold grains for the treatment of choroidal melanoma. Br J Radiol 56:415-420
- Chiu-Tsao ST, Anderson LL, O'Brien K et al. (1990) Dose determination for I-125 seeds. Med Phys 17:815-825
- Cogan DG, Donaldson DD, Reese AB (1952) Clinical and pathological characteristics of radiation cataract. Arch Ophthalmol 47:55-70
- Collaborative Ocular Melanoma Study Group (1990) Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS Report No. 1. Arch Ophthalmol 108:1268-1273
- Coleman KJ, Lizzi FL, Burgess SEP et al. (1986) Ultrasonic hyperthermia and radiation in the management of intraocular malignant melanoma. Am J Ophthalmot 101:635-642
- Cruess AF, Augsberger JJ, Shields JA et al. (1984) Regression of posterior uveal melanomas following cobalt-60 plaque radiotherapy. Ophthalmology 91:1716-1719
- Davidorf FH, Pajka JT, Makley TA et al. (1987) Radiotherapy for choroidal melanoma. An 18 year experience with radon. Arch Ophthalmol 105:352-355
- Donoso LA, Augsburger JJ, Shields JA et al. (1986) Metastatic uveal melanoma. Correlation between survival time and cytomorphometry of primary tumors. Arch Ophthalmol 104:76-78
- Earle J, Kline RW, Robertson DM (1987) selection of iodine-125 for the Collaborative Ocular Melanoma Study. Arch Ophthalmol 105:763-764
- Einhorn LH, Burgess MA, Gottlieb JA (1974a) Metastatic patterns of choroidal melanoma. Cancer 34:1001-1004
- Einhorn LH, Burgess MA, Vallejos C et al. (1974b) Advanced metastatic melanoma - prognostic correlations and response to treatment in 26 patients. Cancer Res 34:1994-2004
- Ferry AP (1964) Lesions mistaken for malignant melanoma of the posterior uvea. Arch Ophthalmol 72:463-469
- Finger PT, Packer S, Svitra PP et al. (1985) Thermoradiotherapy for intraocular tumors. Arch Ophthalmol 103:1574-1620
- Finger PT, Packer S, Paglione RW et al. (1989) Thermoradiotherapy of choroidal melanoma. Clinical experience. Ophthalmology 96:1384-1388
- Finger PT, Moshfeghi DM, Ho TK (1991) Palladium-103 ophthalmic plaque radiotherapy. Arch Ophthalmol 109:1610-1613
- Fitterman HN, McLean JA (1963) Malignant melanoma: a fifteen year review. Am J Ophthalmol 56:90-97
- Flocks M, Gerende JH, Zimmerman LE (1955) The size and shape of malignant melanomas of the choroid and ciliary body in relation to prognosis and histologic characteristics. A statistical study of 210 tumors. trans Am Acad Ophthalmol 55:740-758
- Fraunfelder FT, Boozman FW, Wilson RS et al. (1977) No-touch technique for intraocular malignant melanomas. Arch Ophthalmol 95:1616-1620
- Gomer CJ, Doiran DR, Jester JV et al. (1983) Hematoporphyrin derivative photoradiation therapy for the treatment of intraocular tumors: examination of acute normal ocular toxicity. Cancer Res 43:721-727
- Gomer CJ, Hayashi N, Liu G et al. (1987) Yag laser induced hyperthermia as an adjunctive treatment for ocular melanoma (abstr). Invest Ophthalmol Vis Sci 28:117
- Gomori JM, Grossman RL Shields JA et al. (1986) Choroidal melanomas: correlation of NMR spectroscopy and MR imaging. Radiology 158:443-445
- Gragoudas ES, Goitein M, Verhey L et al. (1980) Proton beam irradiation: an alternative to enucleation for intraocular melanomas. Ophthalmology 87:571-581
- Gragoudas ES, Goitein M, Verhey L et al. (1982) Proton beam irradiation of nveal melanomas: results of a 51/2 year study. Arch Ophthalmol 100:928-934
- Ham WT (1953) Radiation cataract. Arch Ophthalmol 50:618-643
- Jensen OA (1982) Malignant melanomas of the human uvea in Denmark 1943-1952. Acta Ophthalmol 60:161-182
- Karlsson UL, Augsburger JJ, Shields JA et al. (1989) Recurrence of posterior uveal melanoma after Co-60 episcleral plaque therapy. Ophthalmology 96:382-388
- Karolis C, Frost RB, Billson FA (1990) A thin I-125 eye plaque to treat intraocular tumors using an acrylic insert to precisely position the sources. Int J Radiat Oncol Biol Phys 18:1209-1213
- Krishnaswamy V (1978) Dose distribution around an I-125 seed source in tissue. Radiology 126:489-491
- Lagendijk JJW (1982) A microwave heating technique for the hyperthermic treatment of tumors of the eye, especially retinoblastoma. Phys Med Biol 27:1313-1324
- Lean EK, Cohen D, Liggett PE et al. (1990) Episcleral radioactive plaque therapy: initial clinical experience with 56 patients. Am J Clin Oncol 13:185-190
- Lederman M (1956) Radiotherapy in the treatment of orbital tumors. Br Ophthalmol 40:592-610
- Liggett PE, Pince KJ, Astrahan MA et al. (1990) Localized current field hyperthermia: effects on normal ocular tissue. Int J Hyperthermia 6:517-527
- Lincoff H, McLean J, Long R (1967) The cryosurgical treatment of intraocular tumors. Am J Ophthalmol 63:389-398
- Ling CC, Yorke ED, Spiro IJ et al. (1983) Physical dosimetry of I-125 seeds of a new design for interstitial implant. Int J Radiat Oncol Biol Phys 9:1747-1752
- Ling CC, Schell MC, Yorke ED et al. (1985) Twodimensional dose distribution of I-125 seeds. Med Phys 12:652-655
- Ling CC, Chen GTY, Boothby JW et al. (1989) Computer assisted treatment planning for I-125 ophthalmic plaque radiotherapy. Int J Radiat Oncol Biol Phys 17:405-410
- Lommatzsch PK (1983) β-Irradiation of choroidal melanoma with Ru-106/Rh-106 applicators: 16 years experience. Arch Ophthalmol 101:713-717
- Lommatzsch PK (1986) Results after β-irradiation (Ru-106/Rh-106) of choroidal melanomas: 20 years experience. Br J Ophthalmol 70:844-851
- Luxton G, Astrahan MA, Liggett PE et al. (1988a) Dosimetric calculations and measurements of gold plaque ophthalmic irradiators using iridium-192 and iodine-125 seeds. Int J Radiat Oncol Biol Phys 15:167-176
- Luxton G, Astrahan MA, Petrovich Z (1988b) Measurements of backscatter from a single seed of I-125 for ophthalmic plaque dosimetry. Med Phys 15:397-400
- Luxton G, Astrahan MA, Findley DO et al. (1990) Measurement of dose rate from exposure-calibrated I-125 seeds. Int J Radiat Oncol Biol Phys 18:1199-1207
- MacFaul PA, Bedford MA (1970) Ocular complications after therapeutic irradiation. Br J Ophthalmol 54:237-247
- MacFaul PA, Morgan GL (1977) Histological changes in malignant melanomas of the choroid after cobalt plaque therapy. Br J Ophthalmol 61:221-228
- Magnus L (1967) Tiefendosisberechnung für die Co-60 Augenapplikatoren CKA I-4 (nach Stallard). Strahlentherapie 132:379-386
- Markoe AM, Brady LW, Shields JA et al. (1985) Malignant melanoma of the eye: treatment of posterior uveal lesions by Co-60 plaque, radiotherapy versus enucleation. Radiology 156:801-803
- McLean IW, Foster WD, Zimmerman LE (1977) Prognostic factors in small malignant melanomas of the choroid and ciliary body. Arch Ophthalmol 95:4858
- McLean IW, Foster WD, Zimmerman LE et al. (1983) Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol 96:502-509
- Merriam GR, Focht E (1957) A clinical study of radiation cataracts and their relationship to dose. Am J Roentgenol Radiat Ther 77:759-785
- Nakissa N, Rubin P, Stroh] R et al. (1983) Ocular and orbital complications following radiation therapy of paranasal sinus malignancies and review of literature. Cancer 51:980-986
- Naquin HA (1954) Exenteration of the orbit. Arch Ophthalmol 51:850-862
- Nath R, Meigooni AS, Meli JA (1990) Dosimetry on the transverse axes of I-125 and Ir-192 interstitial brachytherapy sources. Med Phys 17:1032-1040
- Newman GH, Davidorf FH, Havener WH et al. (1970) Conservative management of malignant melanoma. Arch Ophthalmol 83:21-26
- Packer S (1987) Iodine-125 radiation of posterior uveal melanoma. Ophthalmology 94:1621-1626
- Packer S, Rotman M (1980) Radiotherapy of choroidal melanoma with Iodine-125. Ophthalmology 87:582-590
- Packer S, Rotman M, Salanitro, P (1984) Iodine-125 irradiation of choroidal melanoma: clinical experience. Ophthalmology 91:1700-1708
- Parsons JT, Fitzgerald Cr, Hood Cl et al. (1983) The effects of irradiation on the eye and optic nerve. Int J Radiat Oncol Biol Phys 9:609-622
- Petrovich Z, Liggett PE, Lean E et al (1990a) Treatment of T3 primary malignant melanoma of the choroid with episcleral radioactive plaque. Endocuriether Hyperthermia Oncol 6:11-17
- Petrovich Z, Liggett PE, Luxton G et al. (1990b) Radioactive plaque therapy in the management of primary malignant ocular melanoma: an overview. Endocuriether Hyperthermia Oncol 6:131-141
- Petrovich Z, Luxton G, Langholz B et al. (1992) Episcleral plaque radiotherapy in the treatment of uveal melanomas. Int J Radiat Oncol Biol Phys 24:247-251
- Petrovich Z, Astrahan MA, Jozsef G et al. (1992) Episcleral plaque thermoradiotherapy in patients with choroidal melanoma. Int J Radiat Oncol Biol Phys 23:599-603
- Peyman GA, Apple DJ (1974) Local excision of choroidal malignant melanoma. Arch Ophthalmol 92:216-218
- Rajpal S, Moore R, Karakousis CP (1983) Survival in metastatic ocular melanoma. Cancer 52:334-336
- Rand RW, Khonsary A, Brown WJ (1989) Leksell stereotactic radiosurgery in the treatment of eye melanoma. Neurol Res 9:142-146
- Riedel KG, Svitra PP, Seddon JM et al. (1985) Proton beam irradiation and hyperthermia. Effects on experimental choroidal melanoma. Arch Ophthalmol 103:1862-1869
- Saunders WM, Char DH, Quivey JM et al. (1985) Precision, high dose radiotherapy: helium ion treatment of uveal melanoma. Int J Radiat Oncol Biol Phys 11:227-233
- Scarborough EC, Koro NC, Antich UT (1988) Substitution of Pd-103 for I-125 in the treatment of choroidal melanomas (abstr). Phys Med Biol 33:128
- Sealy R, Le Roux PLM, Rapley F et al. (1976) The treatment of ophthalmic tumors with low-energy sources. Br J Radiol 49:551-554
- Seddon JM, Albert DM, Lavin PT et al. (1983) A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol 101:1894-1899
- Seigel D, Myers M, Ferris F et al. (1979) Survival rates after enucleation of eyes with malignant melanoma. Am J Ophthalmol 87:761-765
- Shammas HF, Blodi FC (1977a) Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol 95:63-69
- Shammas HF, Blodi FC (1977b) Orbital extension of choroidal and ciliary melanomas. Arch Ophthalmol 95:2002-2005
- Shields JA, McDonald PR (1974) Improvements in the diagnosis of posterior uveal melanomas. Arch Ophthalmol 91:259-264
- Shields JA, Shields CL (eds) (1992) Management of posterior uveal melanoma in intraocular tumors. WB Saunders, Philadelphia, pp 171-194
- Shields JA, Zimmerman LE (1973) Lesions simulating malignant melanoma of the posterior uvea. Arch Ophthalmol 89:466-471
- Shields JA, Augsburger JJ, Brady LW et al. (1982) Cobalt plaque therapy of posterior uveal melanomas. Ophthalmology 89:1201-1207
- Sinclair WK, Trott NG (1956) The construction and measurement of beta-ray applicators for use in ophthalmology. Br J Surg 29:15-23
- Sobanski J, Zeydler-Gredzielewska L, Szusterowska-Martinowa E (1972) Decreased mortality of patients with intraocular malignant melanoma after enucleation of the eyeball followed by orbit x-ray irradiation. Pol Med J 11:1512-1516
- Spencer WH (1975) Optic nerve extension of intraocular neoplasms. Am J Ophthalmol 80:465-471
- Stallard HB (1961) Malignant melanoma of the choroid treated with radioactive applicators. Ann R Coll Surg Engl 29:170-182
- Stallard HB (1966) Radiotherapy for malignant melanoma of the choroid. Br J Ophthalmol 50:147-155
- Thomas JV, Green WR, Maumenee AE (1979) Small choroidal melanomas: a long term follow-up study. Arch Ophthalmol 97:861-864
- Vogel MH (1972) Treatment of malignant choroidal melanomas with photocoagulation. Evaluation of 10 year follow-up data. Am J Ophthalmol 74:1-11
- Weaver KA (1986) The dosimetry of I-125 seed eye plaques. Med Phys 13:78-83
- Weaver KA, Smith V, Huang D et al. (1989) Dose parameters of I-125 and Ir-192 seed sources. Med Phys 16:636-643
- Weinhaus RS, Seddon JM, Albert DM et al. (1985) Prognostic factors: study of survival after enucleation for juxtapapillary melanomas. Arch Ophthalmol 103:1673-1677
- Wright CJE (1949) Prognosis in cutaneous and ocular malignant melanoma: a study of 222 cases. J Pathol Bacteriol 61:507-525
- Zimmerman LE, McLean IW (1979) An evaluation of enucleation in the management of uveal melanomas. Am J Ophthalmol 87:741-760
- Zimmerman LE, McLean IW, Foster WD (1978) Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumor cells? Br J Ophthalmol 62:420-425
Plaque Simulator References |
Guide Contents









