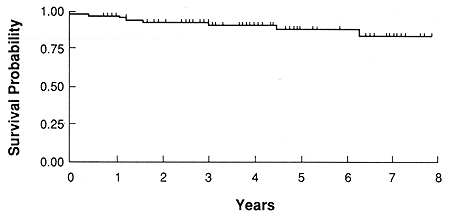
Fig. 1. Probability of survival for all patients
During an 8-year period, 85 patients with uveal melanomas were treated with episcleral plaque radiotherapy (EPRT). The T-stage was: T1 - 3 (4%), T2 - 29 (34%) and T3 - 53 (62%). The mean tumor elevation was 6.1 mm. Radiation dose was prescribed at the tumor apex and at D5mm. The mean D5mm dose was 150.1 Gy (range 70.5-430 Gy) and the mean dose at the apex was 102.6 Gy (range 29.8-200 Gy). Useful vision (> 5/200) was maintained in 73% of patients. The 5-year actuarial survival was 88%. Metastatic disease developed in 9 (11%) patients, 6 of whom died of their disease. Basal tumor dimensions were important factors predicting metastatic disease, p = 0.002. A decrease in tumor elevation was seen in 82%. There was a much lower incidence of decrease in tumor radial and circumferential dimensions, 47.5 and 46%, respectively, p < 0.001. Treatment complications were common (56%), particularly in patients with large tumors (72%), p - 0.04. The incidence of complications was higher in patients treated prior to 1988 as compared to those who were treated more recently (67 vs 35%, p = 0.010). There were 13 (15%) patients who had enucleation. This included 12 treated before 1986 and 1 patient treated subsequently (46 vs 2%, p < 0.001). In a univariate analysis, tumor height and radiation dose at D5mm were important factors predicting enucleation, p - 0.004. In a multivariate analysis, however, the most important factor predicting enucleation was treatment administration prior to 1986, p < 0.001). A sharp decrease in the incidence of severe complications, including enucleation, as seen after 1985, is likely due to a major effort in treatment optimization.
Treatment of patients with uveal melanoma has undergone an evolution. Until the early 1970's, surgical management, consisting primarily of enucleation, was the only widely recognized and effective treatment for these patients (3,6,23). Radiation therapy with episcleral radioactive plaques (EPRT) or particle beams in the past 20 years have become a treatment of equal importance to surgery for T1, T2 and selected T3 lesions (1,3,6,7,13). The attractiveness of radiotherapy is due to its conservative nature. This treatment results in excellent survival with a preservation of useful vision in 50 to 70% of patients (4,13,16,19). External photon beam radiotherapy has also been widely applied as an adjuvant treatment used prior to enucleation in larger T3 tumors. The rationale behind this use of radiotherapy was an attempt to reduce the incidence of postsurgical metastatic disease (8,9,12,23).
In 1985, the Collaborative Ocular Melanoma Study (COMS) was formed. The main objective of this group was to define optimal treatment for patients with uveal melanomas. This was to be accomplished through the use of multi-institutional prospective randomized trials. These trials are well underway and, in several years, they are expected to provide important data to assist ophthalmic oncologists in the selection of an appropriate therapy for patients with choroidal melanoma.
The purpose of this report is to present our clinical experience with EPRT for selected patients with choroidal melanoma.
From 1983 to 1990, 85 patients with choroidal melanoma received EPRT at the University of Southern California School of Medicine (USC). An additional 21 patients were treated in a Phase I trial with episcleral thermoradiotherapy, and 12 patients with medium sized tumors were randomized to COMS EPRT versus enucleation, while 11 were randomized to COMS preoperative radiotherapy versus enucleation alone. Since 1985, only patients who were not eligible for COMS studies or who refused randomization were considered for the University of Southern California based trials.
There were 45 male and 40 female patients. Their ages ranged from 23 to 91 years (mean 63 years). Pretreatment work-up was directed to diagnose choroidal melanoma, to define its precise location and dimensions and to exclude the presence of extraocular tumor extension and metastatic disease. Detailed general and ophthalmic histories and physical examination were performed on all patients. Ophthalmic examination included: visual acuity, fundus photography, biomicroscopy and ultrasound studies using A and B modes. Radiographs of the chest, ultrasound examination of the liver, complete blood count and liver function tests were also obtained. Suspicion of extraocular tumor spread required histological confirmation. In general, pretreatment work-up was similar to that recommended by COMS (10). Since 1987, the routine use of computer axial tomography of the involved eye was implemented to assist in treatment planning (2).
The American Joint Committee recommended staging system for uveal melanoma was used in this report (1). In addition, for comparison, we have also used the COMS recommended system defining patients eligibility for randomized trials and based on tumor dimensions. This COMS system divided tumors until 1990 into small < 3 mm in elevation, medium > 3 mm in apical elevation and/or <=15 mm in basal diameter and large tumors, > 8 mm in elevation and/or >= 16 mm in basal diameter. Most (72%) tumors were posterior to the equator, including 10 (12%) which were juxtapapillary (within <= 1 mm). Anterior tumors were present in 23 (27%) and one (1%) patient had the tumor anterior and posterior to the equator. The majority (62%) of patients had T3, 34% had T2 and 4% had T1 tumors (1). Distribution of patients by T-stage and the COMS classification is shown in Table 1. Mean tumor dimensions were: apical 6.1 mm, radial 12.9 mm, and circumferential 12 min (Table 2).
Visual acuity (VA) was defined as normal reading vision (> 20/50), ambulatory vision (> 5/200) with patients able to read large print with or without corrective measures and nonambulatory vision (< 5/200). In the pre-treatment evaluation, 60 (71%) patients had normal reading vision, 19 (22%) had ambulatory and six (7%) non-ambulatory vision. Patients with large tumors were included in EPRT either because of their refusal of recommended enucleation or due to poor VA in the contralateral eye.
All patients were treated with USC built episcleral 18 Karat gold plaques. Details on these plaques, radiation dosimetry and treatment techniques have been published (2,16,20,21,23). Nearly all patients during the first part of this study were treated with Ir-192, while from 1986 to date, only I-125 was used as the radioactive isotope. A total of 55 (65%) patients received their treatment with I-125 and 30 (35%) with Ir-192. Plaque placements and removals were performed under local anesthesia with treatments given on an outpatient basis.

Radiation doses were prescribed at 5 mm depth and at the tumor apex. The mean D5mm was 150.1 Gy and the mean Dapex was 102.6 (Gy) (Table 3). The policy on prescribed radiation doses has evolved over the years. Following an early analysis of treatment outcome and treatment complications, subsequent to 1985, a D5mm, was kept at <=150 Gy. The apical dose was prescribed following recommendation of COMS at >=100 Gy. This dose of radiation was usually given over a period of 7 days. A major effort has been made to optimize treatment and reduce the dose to the uninvolved critical structures of the eye such as: macula, optic nerve and retina outside the volume of interest (2). Treatment preplanning using the USC developed software system helped to optimize the treatment. Important factors used in this optimization process included: Precise tumor definition, the use of custom episcleral plaques, and the use of I-125 seeds with two to three different isotope activities.
Follow-up examinations were scheduled subsequent to removal of the plaques at 2 and 4 weeks and at quarterly intervals thereafter. The frequency of follow-up visits was reduced to two per year in the second year and to annual visits in the third year and beyond. The first detailed ultrasound examination was performed 3 months posttreatment. The reason for that was a difficulty in the interpretation of echographic findings in the immediate post-implant period. Detailed ophthalmic and general interval history and physical examinations were performed at each follow-up visit. Follow-up ranged from 6 to 96 months with a mean of 37 months.
Statistical analysis included the use of: two sample T-tests, test for trend, and multivariate analysis. Survival was calculated from the day of treatment by the actuarial method.
The overall 5- and 8-year actuarial survival was 88 and 84%, respectively (Fig. 1). There was a decrease in the 5and 8year survival with the increase in the tumor elevation, N.S. at p = 0.14, test for trend (Table 4). Of the 85 treated patients, metastatic disease developed in nine (11%). The first manifestation of metastatic disease was noted in the liver in seven, skin and neck lymph nodes in one patient each. Death due to metastatic disease was seen in 6 patients, while 3 remained alive with distant metastases. Death due to metastatic disease occurred from 5 to 36 months (mean 20.3 months) after treatment. An additional three patients died of intercurrent disease from 3 to 75 months post-treatment.
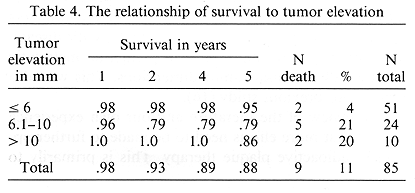
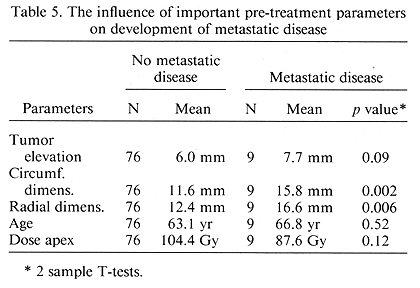
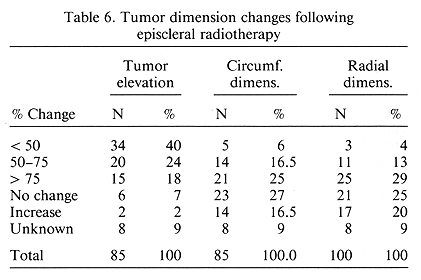
A study was undertaken on the influence of important pre-treatment and treatment parameters on the subsequent development of metastatic disease. Of the several factors examined, basal tumor dimensions had the most important influence on the occurrence of metastatic disease, p = 0.002 (Table 5). A post-treatment change in mean tumor dimensions was examined. As expected, the change seen most frequently was a decrease in apical elevation (Table 6). This decrease was noted in 82% as opposed to a decrease of 47.5 and 46% for the circumferential and radial dimensions ratios, respectively, p < 0.001.
Visual acuity at the last follow-up was: reading 22 (26%), ambulatory 40 (47%), non-ambulatory 10 (12%), and 13 (15%) patients had enucleation.
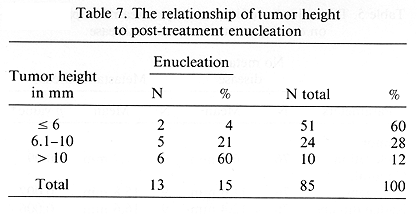
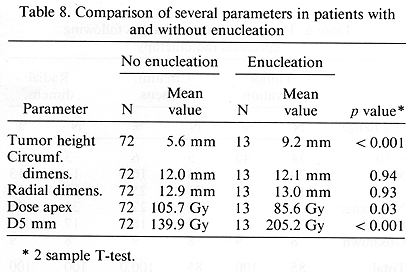
The treatment program was very well tolerated by the study patients. Acute toxicity consisted of mild pain, which was relieved with non-narcotic analgesics. Late complications are of importance since they are responsible for decreased VA and, in extreme cases, result in enucleation. Of the 85 treated patients, 48 (56%) developed late complications. As expected, these complications were more common among the patients with large tumors. Their incidence was 72% in 29 patients with large tumors as compared with 48% in 53 patients with medium sized lesions, p = 0.04. These complications were more common in patients treated prior to 1987 as compared to those treated subsequently (67 vs. 35%, p = 0.010). The most severe complications resulted in enucleation. This was seen in 13 (15%) patients. There was an excellent correlation between tumor elevation and enucleation. This incidence ranged from a low of 4% for tumors < 6 mm in height to 60% in those with lesions > 10 mm in height, p < 0.001 (Table 7). No such relationship existed between enucleation and radial and circumferential dimensions. Enucleation was seen more frequently (27%) in patients who received > 150 Gy, calculated at 5 mm depth as compared to those receiving < 150 Gy at D5mm, p = 0.004 (Table 8). The most striking difference in the enucleation rates was seen in patients treated prior to 1986 and following that year. Of the 26 earlier treated patients, 12 (46%) underwent enucleation and of the 54 later treated patients, 1 (2%) required this procedure, p < 0.0001.
In order to explain this high incidence of enucleation in pre-1986 treated patients, a multivariate analysis was performed. The parameters examined included: tumor dimensions, radiation doses, including D5mm, and Dapex, and T-stage. None of these factors could explain the reduced enucleation rate following 1986. In a logistic regression model, implant year still added significantly in predicting enucleation after controlling for tumor stage and height, p = 0.001. Using the same model dose of radiation did not significantly add in enucleation prediction after controlling for initial tumor height, p = 0.63. The duration of follow-up was not an important factor since all enucleations occurred relatively early (within 27 months of treatment) in this study (Fig. 2). There were however 12 patients whose follow-up has not yet reached 27 months.
The results of this study are similar to the other reports showing the effectiveness of episcleral plaque irradiation in patients with uveal melanoma (4,7,19,22,25). Longterm survival exceeding 80% as seen in our patients has clearly demonstrated the value of this conservative treatment as compared to the surgical treatment alternative. A comparison of treatment results obtained with the use of EPRT and enucleation is currently the subject of a prospective randomized trial conducted by COMS. This important study is likely to provide ophthalmic oncologists with data permitting an objective selection of appropriate definitive therapy in a given clinical situation. An unfortunate problem in this COMS study, until recently however, was a relatively slow patient accrual. This was primarily due to the patients' reluctance to be randomized to a study which had one arm requiring enucleation.
Late complications of EPRT were common in this study and their incidence was similar to other reports (5,15,18,19). In a study of 309 patients with ocular melanoma, Lommatzsch reported the incidence of enucleation in 17.2% and longterm reading vision preservation in 22.7% (19%). The most interesting aspect of our study was the dramatic change in the incidence of serious complications following the implementation of several changes in the treatment program. This was particularly true of a reduction in the incidence of enucleation among the patients treated subsequent to 1985. It appeared that the factors of greatest importance in reducing enucleation rate were: the change of radioactive isotope used for the treatment from Ir-192 to I-125, limiting the radiation dose (D5mm) to <=150 Gy, and the routine use of a treatment optimization program allowing for more selective radiation dose distribution (2,20,21).
Tumor regression was a slow process. It was evident usually after 6 months post-treatment. A decrease in tumor elevation was seen in over 80% of the patients, while change in other tumor dimensions were less apparent. These data are similar to other reports (11,14,15). Likewise, the incidence and sites of metastatic disease are similar to those published in the literature (8,12,19,24).
Survival of patients who develop metastatic disease is very poor. To date, there is no effective systemic therapy which would modify the natural history of this manifestation of choroidal melanoma. No treatment parameters appeared to influence the incidence of metastatic disease. The only factor of importance in predicting metastatic disease was the basal tumor dimensions. This was also seen in our previous study (16).
The review of the literature and our own experience suggest that more efforts need to be made to further optimize radioactive plaque therapy. This is primarily to improve the post-treatment visual acuity and to further reduce the incidence of late treatment complications. Our present efforts in this treatment optimization are directed toward reducing the steep radiation dose gradient between tumor base and its apex and to study the use of adjuvant episcleral hyperthermia (17).