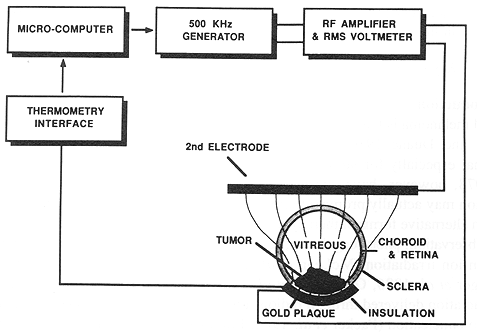
Figure 1. The LCF hyperthermia plaque system.
Using a 500 kHz radiofrequency electromagnetic heating system, the effects of localized current field hyperthermia in normal rabbit eyes were examined. A specially designed scleral plaque placed on normal rabbit eyes was heated to temperatures of 43°C, 45°C, and 47°C for a period of 45 min. The effects of hyperthermia were monitored by clinical examination, fluorescein angiography, electroretinography and histopathology. A graded effect with increasing temperature was found at the lower temperature, and it was confined to the treatment field. At 47°C the electroretinogram was extinguished due to diffuse photoreceptor damage outside the treatment field, as demonstrated by histopathology and electron microscopy. This study indicates that hyperthermia at 45°C for 45 min is the maximum allowable temperature without causing diffuse retinal damage in the normal rabbit eye.
This paper was presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Sarasota, Florida, USA, in May 1989.
Uveal melanoma is the most common primary malignant intraocular neoplasm in adults (Graham and Duane 1980). Enucleation has been the standard treatment for uveal melanoma, especially for large tumours (Zimmerman and McLean 1979). Zimmerman et al. 1978, however, have questioned the benefits of enucleation, and suggested that enucleation may actually promote tumour metastasis. As a result there has been renewed interest in alternative forms of therapy for this condition. Alternative therapies to enucleation include observation, photocoagulation, resection, radioisotope plaque therapy, proton beam, and helium ion irradiation (McLean et al. 1977, Vogel 1972, Peyman and Apple 1974, Augsburger et al. 1986, Gragoudas et al. 1982, Char and Castro 1982). At the present time, irradiation delivered through radioisotope plaque is the most widely used alternative therapy to enucleation (Packer et al. 1984, Lommatzsch 1986, Markoe et al. 1985). The efficacy of I-125 radioisotope plaques vs. enucleation is currently being evaluated by the Collaborative Ocular Melanoma Study (Straatsma et al. 1988). Significant ocular complications occur with irradiation (RT), including radiation retinopathy, optic atrophy, and neovascular glaucoma (Stallard 1966, Packer et al. 1984, Shields et al. 1982).
Hyperthermia (HT) has been shown to have significant synergistic tumoricidal activity when combined with RT at temperatures above 40°C (Lindholm et al. 1987, Petrovich et al. 1989, Manning et al. 1982, Scott et al. 1984, Arcangeli et al. 1980, and Overgaard 1981). The HT-RT combination is currently being investigated in clinical trials conducted by the RTOG and ESHO.
We have developed an instrument that delivers localized current field (LCF) HT from an episcleral plaque for the treatment of intraocular tumours (Astrahan et al. 1987). This device allows for incorporation of RT and HT in a single system, obviating the need for multiple and prolonged procedures. This study evaluated the effects of increasing temperature in an in vivo model using the normal rabbit eye.
The LCF plaque system has been described previously and is shown in figures 1 and 2 (Astrahan et al. 1987). An Apple IIE microcomputer acts as controller for a function generator, a power amplifier, and an eight-channel thermometry system. LCF heating results from the power dissipated by RF currents conducted through tissue between two or more metallic electrodes. A small electrode (the plaque) is placed immediately adjacent to the tumour on the scleral surface, while a second electrode of much larger surface area is placed elsewhere on the body to complete the circuit. The difference in electrode size results in proportionally greater current density near the smaller electrode. Significantly greater local heating occurs there (figure 1).
An eight-channel thermometry interface has been designed using custom thermocouple modules (SensorTek Inc., Clifton, NJ) and DIASI (tm) (Interacive Structures, Bana Cynwood, PA) analogue interface cards (Astrahan et al. 1987). Previous experimental data and the heat dosimetry characteristics have indicated the need for only one thermocouple probe at the geometric midpoint of the plaque surface. Uniform and reproducible heating have obviated the need for intraocular thermometry devices. In addition, these microthermocouples with metallic leads are much less susceptible to selfheating at the low frequency used.
Outbred pigmented rabbits, weighing 2-3 kg, were used in this study. The animals were treated and cared for in accordance with ARVO and institutional guidelines for laboratory animal protection and safety. In keeping with these guidelines, we used a small number (14) of rabbits in this study. Each rabbit was anaesthetized with a mixture of ketarnine (10 mg/kg) and zylazine (10 mg/kg). Topical anaesthesia was achieved with 0.5 % proparacaine. A 180° inferior conjunctival peritomy was performed in the right eye of each animal. The episcleral plaque was sutured into position with the anterior border 3 min from the corneoscleral limbus using 6-0 vicryl. The plaque position was marked on the scleral surface with unipolar cautery. A control group of two animals (two eyes) had only the plaque in place with no HT. Three other groups had LCF HT applied for a period of 45 min. Three temperatures, 43°C, 45°C and 47°C, were evaluated to determine the effects of therapeutic HT on the rabbit eye. Four rabbits (four eyes) in each of the other three groups were subjected to each temperature (43, 45, and 47°C for a total of 12 animals or 12 eyes. One rabbit in each temperature group was sacrificed immediately, and the treated eye enucleated. The other nine animals were followed for a period of either 1 or 4 weeks with eyes being enucleated immediately following sacrifice. The control group, consisting of two rabbits, was monitored for 4 weeks. This monitoring was accomplished by clinical examination using biomicroscopy, indirect ophthalmoscopy, colour fundus photography and fluorescein angiography. Photopic and scotopic electroretinography (ERG) were performed immediately prior to sacrifice in both eyes of all animals. Enucleated eyes were fixed in 1/2 Karnovsky's solution and placed in 0.1 M phosphate buffer. The samples were dehydrated in graded ethanols, ending in absolute ethanol, embedded in paraffin and sectioned with Sorvall microtome. Serial step-sections were obtained inside and away from the treated field. Sections (31μm) were stained with haernatoxylin and eosin. Selected samples were processed for transmission electron microscopy (EM). Sections (1 μ) were put on MT2 Sorvall Microtome. Thin sections were stained with 4% uranol acetate in 50% methanol and lead citrate, and viewed on a 50 μm mesh using a Zeiss EM 10-A.
External evaluation of the 12 treated animals immediately following HT showed a graded amount of inflammation of the conjunctiva and sclera compared with the two control animals. Mild inflammation was seen in four eyes treated at 43°C. This inflammatory effect increased in a step-wise fashion with severe inflammation and oedema noted at 47°C (figure 3). This inflammation subsided in all 12 eyes treated with HT. The inflammation resolved without treatment in eight eyes treated at 43°C and 45°C over 7-14 days. However, in the four eyes treated at 47°C the inflammation was noted for up to 4 weeks and required topical steroids to assist in the resolution of the inflammation. No residual effects were noted in the eight animals treated at 43°C and 45°C, but significant conjunctival and lid scarring occurred in the group of three rabbits treated at 47°C. The corneas and lenses of all animals remained clear throughout the monitoring period.
Indirect ophthalmoscopic examination demonstrated a range of minimal to marked retinal oedema in the treatment field dependent on temperature compared with the control group, which was normal (figure 4). This phenomenon was restricted to the treatment field, and the retina appeared normal outside this region in all animals. The retinal oedema resolved after 7 days by clinical examination without sequelae in the eight eyes treated at 43°C and 45°C. The four eyes treated at 47°C were noted to develop chorioretinal scarring with RPE hyperplasia in the treatment field at 4 weeks (figure 5).
Fluorescein angiography was normal at 4 weeks in the two eyes in the control and in the eight treated eyes at the temperature of 43°C and 45°C, both in and outside of the treatment field. A lack of choroidal perfusion was seen in the treatment field in the four animals at 47°C. This persisted up to 4 weeks following treatment. Additionally, there was a marked, late hyperfluorescence observed in the treatment field (figure 6).
ERG performed at 4 weeks in the eight eyes treated at 43°C and 45°C showed no significant difference vs. the untreated fellow eyes or the four eyes in the two animals of the control group. The ERG was extinguished in all three eyes at 4 weeks treated at 47°C. This result indicated a diffuse loss of organized retinal function (figure 7).
Light microscopy demonstrated mild to moderate retinal atrophy confined to the treatment field with retention of the normal retinal architecture at I and 4 weeks posttreatment in the 43°C and 45°C groups (figure 8). The four eyes treated at 47°C showed marked intercellular oedema with dropout of photoreceptor outer segments 24 h following treatment. Marked retinal necrosis and scarring were noted in the treatment field at 1 and 4 weeks following treatment (figure 9). These effects were not seen outside the treatment field in any of the eyes.
Transmission EM demonstrated photoreceptor degeneration up to 5 mm outside the treatment field in animals treated at 47°C, corresponding to their lack of retinal function. The photoreceptors in the eight animals treated at 43°C and 45°C were found to be intact outside the treatment field (figure 10).
Hyperthermia is a viable adjunctive modality for treatment of intraocular neoplasms (Astraham et al. 1987, Riedel et al. 1985, Lagendijk 1982, Finger et al. 1985). In addition to its primary cytocidal properties, HT produces a synergistic effect when combined with RT (Arcangeli et al. 1980, Overgaard 1981). These effects are well known for a variety of tumours, and are now being investigated for choroidal melanoma.
Several studies have shown that HT alone can cause complete regression of malignant tumours. The HT effect is related to both temperature and its duration (Dewhirst et al. 1982). The optimal temperature range to achieve this hyperthermic effect is about 42-43°C (Sapareto and Dewey 1984). At temperatures above 44°C thermal damage occurs rapidly, and any selective sensitivity of malignant tissues may be lost. HT and RT show additive and synergistic effects on cells in different stages of mitotic division (Suit and Gerwick 1979, Arcangeli et al. 1980, Overgaard 1981). Additionally, tumours may preferentially absorb heat. The ischaemia induced by HT leads to relative hypoxia and lowered pH in the tumour compared to normal tissue (Overgaard 1981).
Three methods are currently available for intraocular heat delivery, i.e. ultrasound, microwave, and LCF. Of these, only microwave and LCF are amenable to episcleral plaque therapy. Scleral plaques for HT offer the advantage of good localization and potential for sparing of normal uninvolved structures (Finger et al. 1985, Lagendijk 1982, Astrahan et al. 1987). Maximal heat is delivered to the base of the tumour where the tumour vasculature is located. The inefficient vasculature of neoplastic tissue leads to preferential thermotoxic effects on the tumour. An additional major advantage of this plaque therapy is the ability to combine both RT and heat delivery systems in one device. This allows for simplification of the procedure, requiring surgery only once.
The main treatment goals in patients with uveal melanoma are local tumour control and prevention of death from metastatic disease. Preservation of vision is an important secondary goal. Radiation or enucleation is the primary mode of therapy (Markoe et al. 1985, Augsburger et al. 1986). HT is an adjunctive measure to be evaluated in patients with tumours > 8 mm in elevation. It is hoped that the use of adjuvant HT will result in an increased incidence of tumour control without an increase in treatment toxicity. If this is true, in the future it may be possible to reduce RT dose, thus reducing the high incidence of radiation complications without compromising the incidence of tumour control. Normal areas immediately adjacent to the tumour are at risk for toxic effects of localized HT. A small treatment margin usually 2 mm around the tumour may be necessary to prevent tumour recurrence.
The optimal HT delivery system should provide a uniform heating in a reproducible manner. The effect of treatment should be localized to allow sparing of normal adjacent tissue of any significant toxicity. We have shown a localized hyperthermia effect using the LCF system. When temperatures were maintained at 45°C or less there were some retinal changes in the treatment field, but not abnormalities elsewhere. The RPE also exhibited mild aberrations, but no marked hyperplasia or chorioretinal scarring. No haemorrhages, effusion or retinal detachments were noted. ERGS indicated normal retinal function in animals treated at these temperatures. Significant toxicity, however, occurred in four eyes treated at 47°C. There was retinal necrosis and chorioretinal scarring in the treatment zone.
The HT system utilized in this study is of low cost, uses existing technology and the simplicity of plaque-electrode construction makes a wide variety of custom shapes and sizes possible. At a radiofrequency of 500 kHz tissue behaves more like a conductor than a dielectric, resulting in diminished electric field attenuation and greater heat penetration. The localized heat effect and the minor toxicity of this modality, when used at a temperature < 45°C, should allow preservation of function while delivering therapeutic temperature at the tumour apex (Astrahan et al. 1987). The rabbit model differs from humans in that the rabbit retina is supplied mainly by choroidal vessels with very small retinal vascular contribution. There is little, if any, cooling capacity from retinal vessels. By contrast, human retina is supplied from both retinal and choroidal sources. The additional cooling capacity of the retinal circulation in humans might help minimize the effects of hyperthermia on normal tissue; thus LCF hyperthermia may prove less toxic to human retina.
Further investigation of heat distribution in an eye tumour model system is still required to characterize heat dosimetry in tumour tissue. Tumoricidal activity of LCF hyperthermia alone, and in combination with radiation, must also be examined.
The findings of this study suggest that 45°C is the limiting temperature of hyperthermic treatment using LCF HT in episcleral plaque. Temperatures above 45°C may be associated with severe and unacceptable toxicity and diffuse damage to the photoreceptors.