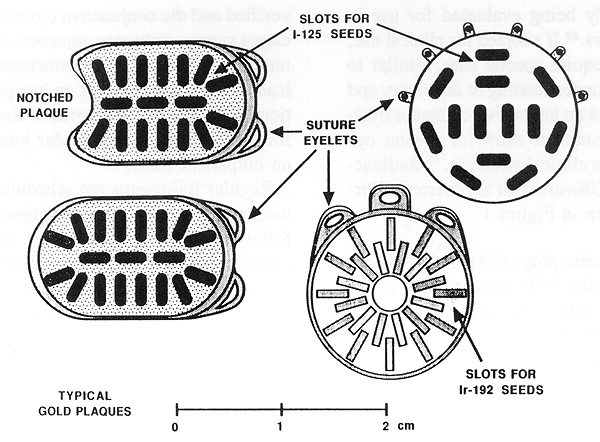
Figure 1. Radioactive plaques commonly used at USC and an example of cooperative ocular Melanoma study (COMS) plaque in the right upper corner.
Primary tumors of the eye are uncommon. There are fewer than 2000 new cases diagnosed annually in the United States (1). Primary malignant melanoma of the uveal tract (MMUT) accounts for approximately 80% of all primary eye tumors and 6% of all melanomas (1,2). The incidence of MMUT in the United States is 0.6 per 100,000 population (2). This tumor has been reported to be more common among past-middle-aged white men, with its peak incidence occurring in the sixth decade of life (2). Callender et al. (3) in a study of 1600 patients with MMUT found a male-to-female ratio of 1.06 and a strong racial preponderance favoring whites.
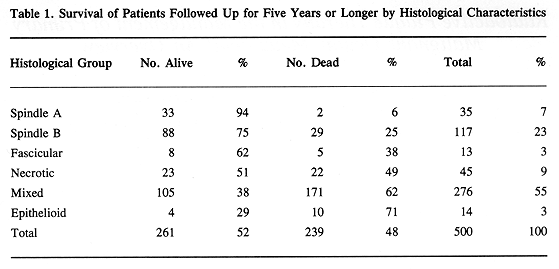
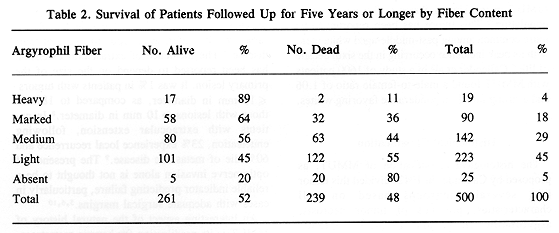
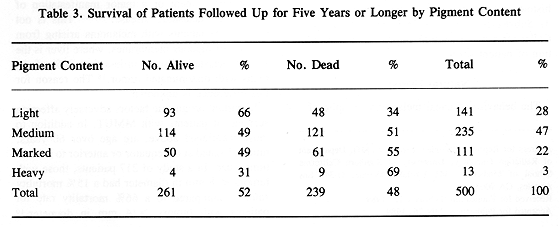
The histological classification of MMUT, as proposed by Callender in 1942, divided this tumor into several subgroups based on cell characteristics, presence of tumor necrosis, argyrophilic fiber, and pigment content (3). Histological groups based on cell characteristics were spindle A and B, fascicular, epitheloid, and mixed. There was a good correlation between histological pattern and survival (Tables 1 through 3). Subsequently, in 1970 McLean et al. (4) modified Callender's classification, improving the prediction of patient survival.
The behavior of uveal melanoma, in spite of numerous retrospective reports, is not completely understood. It is generally believed that the tumor growth is initially local with gradual invasion of neighboring structures, such as optic nerve or sclera (5). Tumor cells can also be found in the vitreous, particularly in patients with larger or necrotic tumors (6). Vitreous involvement is a grave prognostic sign, since 83% of the patients with this tumor manifestation were reported to die of their disease (7). The incidence of extrascleral extension has been reported to depend on the size of the primary lesion. It was 1% in patients with tumors <= 10 mm in diameter, as compared to 18% in those with lesions > 10 mm in diameter (8). In patients with extraocular extension, following enucleation, 23% experience local recurrence and 60% die of metastatic disease (9). The presence of optic nerve invasion alone is not thought to be a reliable indicator predicting failure, particularly in cases with adequate surgical margins (5,6,10).
An interesting aspect of the natural history of MMUT is its predilection for hepatic metastases. In patients with metastatic choroidal melanoma, 50% to 90% had liver as the only site of metastatic tumor involvement or a major manifestation of multiorgan metastatic disease (5,11-15). This is not the case in patients with melanoma arising from the skin or mucous membranes, where liver is the site of metastatic disease in less than 20% of patients with disseminated tumor (16) The reason for this difference is unknown.
Important prognostic factors adversely affecting survival of patients with MMUT, in addition to those mentioned above, are age over 60 years, tumors located at the equator or anterior to it, (7) and tumor size. In a study of 217 patients, those with tumors < 8 mm in diameter had a 15% mortality rate, as compared to a 66% mortality rate for patients with tumors > 14 mm in diameter (10). Similar findings were reported by other investigators (17).
Symptoms and signs in patients with choroidal melanoma depend on the tumor size and its location. In one report blurred vision, decreased vision, and ocular pain were the most frequent symptoms, while retinal detachment, presence of a mass, and intraocular hemorrhage were the most frequent signs (18).
Diagnostic workup is directed toward the establishment of a diagnosis of MMUT, characterization of the tumor in detail, and general systemic assessment to rule out the presence of metastatic disease. Diagnosis of MMUT is usually made using an indirect method without pretreatment histological confirmation of malignant melanoma. The following steps are taken: detailed general history; detailed ophthalmic history; ophthalmic physical examination; visual acuity; fundus photography; biomicroscopy noting tumor location and its characteristics; and ultrasonographic examination using A and B scan. Because of the critical importance of ultrasound in establishing a diagnosis of MMUT, this examination should be performed by an ophthalmic ultrasound specialist. Additionally, in some centers, fluorescein angiography is a routine part of the workup.
Systemic evaluation consists of detailed, complete physical examination; radiograph of the chest: posterior-anterior and lateral; complete blood cell count; serum alkaline phosphatase value; and serum lactic acid dehydrogenase value. This basic systemic evaluation was found to provide reliable information on the presence of metastatic disease in nearly all patients (16). The presence of an abnormality detected in the history, physical examination, or laboratory studies requires additional tests. Needle biopsy is required to confirm the presence of metastatic disease.
In the past, an accurate diagnosis of malignant melanoma was made in 80% of patients (19,20). In the remaining 20% of patients the diagnosis was incorrect owing to the presence of lesions simulating choroidal melanoma and failure of the use or proper interpretation of all diagnostic tests. In a recent study of 1398 consecutive enucleations at Wills Eye Hospital, the incidence of incorrect diagnoses was 3.7%. In the final part of this study consisting of 103 enucleations, this incidence was further reduced to less than 2% (21).
Primary malignant melanomas of the choroid can be divided into three groups as recommended by the Collaborative Ocular Melanoma Study Group:
|
|
Small tumors <= 3 mm in height and up to 5 mm in diameter. |
|
|
Intermediate tumors 3.1 mm to 8 mm in height and up to 16 mm in diameter. |
|
|
Large tumors > 8 mm in height and/or > 16 mm in diameter. |
The American Joint Committee recommended a new TNM staging system for uveal melanoma (22):
|
|
Tumor <=10 mm in diameter and <=3 mm in height. |
|
|
Tumor >10 mm <=15 mm in diameter and >3 mm to <=5 mm in height. |
|
|
Tumor > 15 mm in diameter and > 5 mm in height. |
|
|
Tumor with extraocular extension. |
|
|
No regional lymph node metastasis. |
|
|
Regional lymph node metastasis. |
|
|
No distant metastasis present. |
|
|
Distant metastasis. |
|
|
T1, N0, M0 |
|
|
T2, N0, M0 |
|
|
T3, N0, M0 |
|
|
T4, N0, M0 |
|
|
T, N1, M0 |
|
|
T, any N, M1 |
Several modalities have been used in the treatment of MMUT. The most commonly used was surgery, with a more recent trend favoring radiotherapy. Other treatments include photocoagulation, cryosurgery, photoradiation, and hyperthermia.
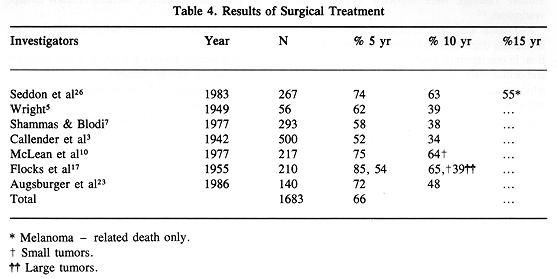
Surgical treatment is directed toward removal of the entire tumor. Unfortunately, with few exceptions, it requires enucleation (2,7,8,18,23-25). In highly selected patients with smaller tumors, local excision can be successfully applied preserving vision (28). Larger tumors, and those with extrascleral extension, usually require a more radical surgical procedure for tumor control (8,9,29). Properly applied surgical treatment results in excellent survival rates well above 60% at five and ten years (Table 4).
In the late 1970s, some investigators (10,31) raised an important question: Does enucleation increase the incidence of metastatic disease in patients with MMUT? Because of the absence of randomized studies comparing surgical treatment with radioactive plaque therapy (RPT), this important problem has not been resolved. To minimize the possibility of tumor cell dissemination during a surgical procedure, a "no-touch" technique has been proposed (32). Other investigators recommended the use of adjuvant external beam radiotherapy to accomplish the same goal (33). Recently, a randomized trial was begun by the Collaborative Ocular Melanoma Study Group. This trial may help find answers to these important management problems.
Cryosurgical treatment was proposed in the past to manage patients with ocular malignancy, including MMUT (34). This treatment, however, is no longer widely used and is thought to be of only palliative or adjuvant value.
Photocoagulation or the use of diathermy in management of MMUT at this time is largely of historical interest and is not widely used (35,36). The use of photoradiation therapy following the administration of hematoporphyrin derivatives has been investigated. Preclinical studies seem to indicate the possible usefulness of this modality in the management of highly selected patients with ocular malignancy (37).
Hyperthermia in combination with RPT or proton beam teletherapy is a promising new treatment modality for patients with ocular tumors (38-42).
Particle beam radiotherapy has been used effectively to treat patients with MMUT. Proton beam irradiation can even be used to successfully treat patients with large tumors (43,44). The use of helium ion beam resulted in excellent tumor control rates (45-47). A major problem with the use of particle beam radiotherapy is the very high cost of treatment instrumentation, which makes wide use of this treatment impossible.
External beam photon irradiation, as a primary treatment for MMUT, has limited application (48). It is an excellent palliative tool for locally advanced tumors and a useful adjuvant therapy preceding or following a surgical procedure.
Radioactive plaque therapy has been used for many years as a valuable treatment alternative to enucleation (49-59). This wide use of RPT was made possible by a cobalt-60 plaque designed by Stallard (49) and subsequently popularized in this country by Rotman (50,51) and by Brady and Shields (57,58). Rotman of New York Medical College also deserves credit for an early work on RPT dosimetry. Other plaques were designed using radioactive iridium-192, iodine-125, and Ru-106/Rh-106 (51,53,60). The main advantage in using I-125 plaque is radiation protection. (Figure 1).
Radioactive plaque dosimetry. Luxton et al. evaluated dosimetry of commonly used radioactive plaques, including those containing Co-60, Ir-192, and I-125 (60,61). The central axis dose rates were found to be essentially the same for tumors of up to 10 mm in height (Figure 2). A substantially more precipitous rate of dose fall-off can be obtained using beta-ray applicators, such as Sr-90/Y-90 and Ru-106/Rh-106 (53,61). Selective use of these beta applicators may result in more optimal protection of the uninvolved normal structures, such as macula, optic nerve, or lens of the eye. A major drawback of these applicators is that they are generally not suitable for the treatment of tumors > 3 mm in height.
Radioactive plaque dosimetry is a complex problem. Absolute dosimetry for Co-60 and Ir-192 has been relatively well understood. This included recent reports on dose measurements in the close proximity of Ir-192 seeds (60,62,63). On the other hand, until recently, dosimetry problems of I-125 plaques have been poorly understood. Measurements and calculations have indicated dose rate from exposure calibrated I-125 seeds have been overestimated by approximately 10% (63,64,65). The presence of gold foil affects I-125 dose rate by reducing its relative full scatter by 6% to 10%, depending on the distance (66,67). This observation is not accepted by all investigators (68). These dosimetric problems with I-125 are due to the complexity of low-energy x-ray interactions.
A new radioactive isotope, palladium-103, with a gamma-ray energy of 21 KeV and a half-life of 17 days, is currently being evaluated for use in ophthalmic applicators (69). If adopted for clinical use, its dosimetry will require special care, similar to that of I-125. A better understanding of dosimetry and improved modeling in an interactive computer treatment planning environment allow for greater optimization in a given clinical situation (70). Radioactive plaques used at University of Southern California (USC) are shown in Figure 1.
Technique of radioactive plaque. At USC, radioactive plaques containing Ir-192 were used prior to 1986. Subsequently, only I-125 plaques have been used (60,66,71) (Figure 1). The reason for this change was the superior radiation safety of I-125 as compared with Ir-192.
Plaque placement is accomplished as an outpatient procedure. Patients are brought to the operating room and intravenous sedation is given. This is augmented with a retrobulbar and eyelid block to obtain local anesthesia and akinesia. The conjunctiva is opened at the limbus centered around the location of the lesion. Subsequently, Tenon's capsule is dissected off the sclera. The rectus muscles are isolated, and 2.0 traction silk sutures are placed beneath each muscle. The eye is then carefully examined with a combination of indirect oplithalmoscopy and transillumination. The tumor borders are identified and appropriate marks are placed on the sclera, using a Mira diathermy instrument. A nonradioactive plaque, identical to the radioactive plaque to be used in the procedure, is sutured into position over the scleral marks. To ensure proper plaque placement, the eye is again examined, using indirect oplithalmoscopy and transillumination. In the case of a need for plaque position adjustment, the sutures are replaced. The radioactive plaque is then sutured into the sclera overlying the tumor. The plaque position is again verified and the conjunctiva closed, using 6-0 plain catgut suture. Subtenon injection of 20 mg of gentamicin and 40 mg of dexamethasone is given. A lead shield is placed over an eye patch and the patient is discharged. Removal of the implant is performed with the patient under local anesthesia on an outpatient basis.
Regular follow-ups are scheduled at 1, 3, and 6 weeks and every 3 months thereafter. At each follow-up, the patient is to undergo careful oplithalmologic examination, similar in complexity to the pretreatment examination. At quarterly intervals, patients undergo ophthalmic ultrasound examination using A and B modes.
All radiation doses were prescribed on the central axis and the scleral dose at 1-mm depth (inner surface of the sclera). Radiation dose was prescribed to be 80 to 120 Gy at the tumor apex given over an average period of seven days. A 2-mm margin around the base of the lesion was to receive the same dose as the tumor apex. After the experience with several patients with tumors > 10 mm in height who developed treatment complications following administration of radiation dose > 200 Gy at 5-mm depth a dose reduction was introduced. A new dose was not to exceed 200 Gy at 5 mm or preferably <= 160Gy. A detailed description of radioactive plaques used at USC has been published (60,64,66,71).
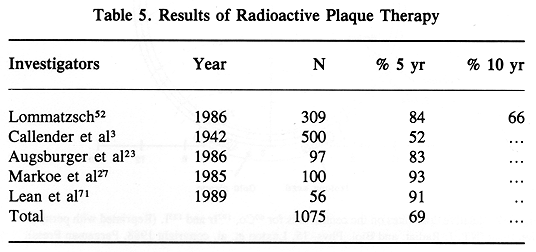
Treatment results in patients given RPT have been excellent. Generally, the published reports show survival rates equal or better to those following enucleation with preservation of vision in most treated patients (23,27,49,50,52,56) (Table 5).
Management decisions in patients with MMUT are based on a number of retrospective studies. The absence of prospective randomized trials frequently makes a treatment decision in a given patient difficult. The most frequent dilemma of ophthalmic oncologists is deciding what is the best management for lesions < 8 mm in height. In some medical centers, enucleation is the treatment of choice for these lesions, while in others it is radioactive plaque or particle beam radiotherapy. The value of adjuvant external beam radiotherapy in patients with tumors > 8 mm in height, or lesions with large basal diameter, is frequently discussed. Although preoperative radiotherapy has been routinely used in many medical centers for patients with large tumors, there is no proof of its value.
In an attempt to provide answers to these important management questions, as well as to prospectively collect valuable data on tumor behavior, patterns of failure, and treatment complications, in 1985 a major multiinstitutional study group was formed. The Collaborative Ocular Melanoma Study Group has been conducting three prospective studies:
From the beginning of these studies, it is expected that in five to ten years, important management information will be available to clinicians. Treatment decisions in patients with MMUT will be based on sound data.
A large number of patients have been receiving radiotherapy for head, neck, and brain tumors. Frequently, either the entire eye or its parts are exposed to therapeutic doses of radiation. The effects of these therapeutic doses on the eye have been well studied (72-78). Radiation injuries of significance, such as major retinopathy or substantial optic nerve injury, tend to occur among patients who have received > 60 Gy (76,77). It should be remembered, however, that of the patients receiving this high dose of radiotherapy, only one third is expected to develop clinically important complications (74). The incidence of ocular complications is higher among patients receiving chemoradiotherapy combination than in patients receiving radiotherapy alone (74).
Acute complications of RPT are limited to an occasional case of mild local infection. In the USC clinical experience with more than 80 RPT patients, no delay in healing or plaque displacement was noted. The use of local anesthesia for plaque placement and its removal has been well tolerated and enthusiastically accepted by patients, since it does not require hospitalization. Clinically significant complications of RPT are limited to late effects of radiotherapy. The overall incidence of these complications is reported to be up to 40% (49,51,57,73). The most commonly observed complication is perimacular exudate, which tends to resolve slowly (49). Retinal and vitreous bleeding and vascular changes leading to retinopathy are also common (49,73). In a study published by Packer et al. (51) the overall incidence of late complications was 34%. Retinopathy, vitreous hemorrhage, cataract, and glaucoma were the most frequent complications. Shields et al. (57) reported 40% of the complications in a study of 100 patients treated with Co-60 plaque. In 23% of the patients in this study, late complications resulted in a substantial decrease in visual acuity. Serious complications requiring enucleation occur in approximately 10% of treated patients (49).
The incidence of complication depends on numerous factors, which include original tumor volume, total dose of radiation, tumor location, and likely radioactive isotope used for the treatment. It is expected that the incidence of complications will be lower among patients treated with plaques containing I-125, as compared with those treated with Co-60.
A review of the data on RPT complications suggests good treatment tolerance by most patients and an acceptable level of late sequelae. In the majority of patients, the tumor can be eradicated with preservation of useful vision. This information tends to bias most patients to accept this form of therapy over enucleation.
The fundamental objective of RPT is to control ocular tumors while retaining useful vision. Present techniques have high complete response rates for tumors < 8 mm in height, but late vision-impairing complications negate some of these advantages. It is possible that these late complications may result from radiation exposure of uninvolved ocular tissues. Future developments in ocular plaque therapy should be directed toward reduction of radiation dose to uninvolved ocular structures and finding adjuvant techniques to enhance the radiation sensitivity of the tumor. This may permit more effective treatment of larger tumors.
The recent availability of low-cost I-125 seeds has opened a new era in ophthalmic plaque therapy. Although there are still some uncertainties in the absolute dosimetry of I-125 because of the low energy and source construction, the advantages of this isotope are compelling. Dose distributions in the tumor volume from I-125 plaques are equivalent to those obtained with Co-60 or Ir-192 (60) but deliver a significantly lower dose outside the tumor volume compared to those earlier isotopes. Exposure to surrounding personnel is greatly reduced. The low energy of I-125 permits nearly complete attenuation of any non-tumor-directed photon flux using only a thin gold shell, enabling highly effective collimation of the flux pattern around a plaque. This collimation could be employed to further reduce dose outside the tumor volume. To clinically implement this capability, however, accurate dosimetry and three dimensional treatment planning capability are being developed (60,64,66,70,79).
Plaque dosimetry differs from more conventional brachytherapy dosimetry in that the entire region of interest lies within 3 cm from the plaque, with the most important region lying within 1 cm. Under these circumstances, seeds cannot be considered as point sources, a practice common to conventional dosimetry systems. Furthermore, the low energy of I-125 increases the importance of photoelectric absorption, and thus the effective Z of the irradiated media. An ophthalmic dosimetry system must treat I-125 seeds as linear sources and take into account source anisotropy, tissue attenuation, and scatter, including attenuation in material intervening between the sources and the eye (such as silicon rubber) and collimating effects of the gold shell. An interactive, three-dimensional dosimetry system has recently been described by Astrahan et al. (70,79). Accurate dosimetry around a plaque, however, does not constitute a treatment planning system. Treatment planning also requires accurate determination of tumor size and location. The present approach is to use a collage of fundus photos to determine the tumor periphery on the retina relative to the optic disc and macula and ultrasonography to measure tumor height. Such a system is in the final stage of development at USC. A potentially superior approach would be to use computed tomography and/or magnetic resonance imaging to directly measure the specific three-dimensional anatomy for each patient prior to plaque placement (for treatment planning purposes) and again following plaque placement to verify plaque location and orientation. Magnetic resonance imaging is a particularly attractive modality for this development, since the gold plaques do not produce any undesirable artifacts. Accurate three-dimensional patientspecific anatomy and verification of plaque position could, in turn, lead to automatic optimization of plaque design, taking into account source location, activity, orientation, and shell design. This capability would be particularly useful for tumors that lie adjacent to the optic nerve, lens, and macula or that have asymmetrical shapes. Such an optimizing treatment planning system might also design and machine wax models of customized plaques, which could then be cast in gold.
In addition to improvements in radiation dosimetry and treatment planning, the adjuvant use of hyperthermia with ophthalmic plaque therapy is currently being explored (38-42). Hyperthermia may prove useful in controlling tumors exceeding 8 mm in height by enhancing the effectiveness of radiation dose. Ultimately, it might also lead to an eventual reduction in the radiation dose prescribed for smaller tumors. This, in turn, could result in the reduction of late radiation complications. It remains to be determined, however, what acute and late complications are associated with the hyperthermia, and how to quantify a "thermal dose." Any additional complications associated with hyperthermic treatment must be balanced against any reduction in radiation complications.
Several ophthalmic hyperthermia systems have been described in the literature. Lagendijk (38) has described a 2450MHz microwave stripline applicator, which is particularly useful in the treatment of retinoblastoma (38). A focused ultrasound system has been described by Lizzi et al. (80) for use in conjunction with proton beam therapy. Gomer et al. (81) are pursuing the use of low-power continuous-wave Nd:YAG laser as a source of highly localized heating. The above techniques are best suited to external beam type radiotherapy.
Finger et al. (39) have described a 5.8-GHz microwave patch resonator mounted in a plaque that permits concurrent irradiation and heating. The steep spatial temperature gradient (about -1°C/mm) in "front" of this device limits its usefulness for larger tumors. Astrahan et al (40,41) have described a technique that also permits concurrent irradiation. Gradients of less than -0.5°C/mm were measured in front of this device in normal rabbit eyes. The technique uses the concave side of a slotted gold plaque as one electrode in a 500-kHz localized current field (LCF) system. The I-125 seeds are glued into slots with a cyanoacrylate adhesive, and removable microthermocouple thermometry is inserted into an additional slot. Temperature over the plaque surface is relatively homogeneous, with about 0.5°C higher temperatures at the edges of the plaque. It is desirable in hyperthermia to slightly enhance heating at the periphery of a tumor to compensate for conductive cooling. The plaques may be any shape or size. At USC, we have developed confidence in the predictability of temperature distribution in the eye, and invasive thermometry is not in routine use. The temperature is carefully monitored on the surface of the plaque (40,41). Invasive thermometry measurements were obtained in two patients. Controlling the surface temperature at 45°C gave a temperature of 42°C at the tumor apex (10-mm depth).
The convenience of plaque-based heating as an adjunct to plaque brachytherapy is evident. The major goal for ocular hyperthermia will be to improve control in the larger tumors. Further evolution of the plaque-based microwave techniques will involve improving thermal homogeneity in large tumors. Future developments in LCF technology will address the dosimetric desirability of offsetting the sources by about 1 mm from the scleral surface while retaining the electrical properties of the plaque. New plaque designs with multiple concentric electrodes would eliminate the need for a second large electrode elsewhere on the body.
The usefulness of hyperthermia. as an adjuvant to plaque brachytherapy will be enhanced by the development of hyperthermia treatment planning that can predict temperature distributions within the eye. In general, hyperthermia treatment planning is complicated by the difficulty in measuring patient specific anatomy and blood flow characteristics. The eye is an ideal site for such a treatment planning system, having a well-defined and consistent structure. In addition, the eye has low blood flow in all but the choroid, and no blood flow in the vitreous. This greatly simplifies the modeling complexity. A treatment planning system with a highly interactive, three dimensional graphics-based user interface, and which combines both radiation and hyperthermic dosimetry, is presently under development at USC. This system is being implemented on a lowcost microcomputer workstation that will enable wide distribution of the system. The imminent availability of low-cost, accurate treatment planning for plaque therapy should greatly improve the quality of care for patients with ophthalmic tumors.