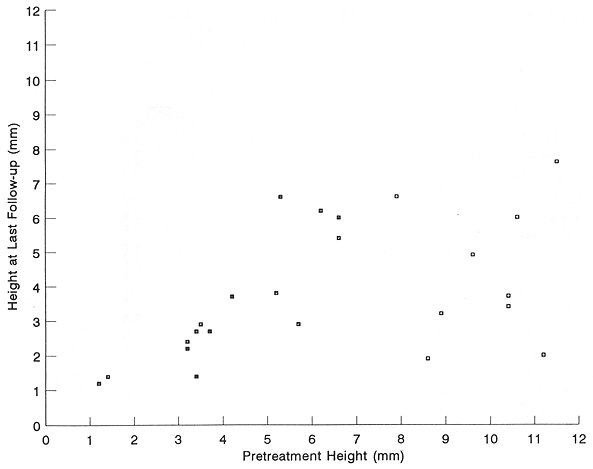
FIG. 1. Tumor height reduction following the administration of episcleral thermoradiotherapy.
Episcleral plaque radiotherapy is a widely applied treatment for selected patients with uveal melanomas. This treatment is well tolerated but may produce severe late radiation complications resulting in decreased visual acuity that reduces the attractiveness of conservative therapy. The purpose of this study was to assess if the addition of episcleral hyperthermia decreases late radiation complications through radiation dose reduction while maintaining high incidence of local tumor control. In a 3-year period, episcleral plaque thermoradiotherapy was given to 25 patients with uveal melanoma in a Phase I study. The mean tumor height was 6.2 min and the mean tumor basal area was 173 mm². The mean radiation dose given to the tumor apex was 72.2 Gy and the mean hyperthermia temperature, given once for 45 min, was 43.5°C. Of the 25 patients treated, 22 (88%) showed tumor height reduction, 2 (8%) showed no change, and 1 (4%) had an increase in tumor height. At the last follow-up (range, 20-68 months; mean, 31.2 months), a 43% mean tumor height reduction was recorded (p = 0.0002). Of the 22 patients initially showing tumor regression, 2 (9%) had subsequent tumor progression. At least ambulatory vision (>5/200) was maintained by 20 (80%) patients. Severe complications, including hemorrhagic retinal detachment and a large vitreous hemorrhage, were seen in 2 (8%) patients early in this Phase I study. The treatment program was well tolerated by the study patients. Severe late treatment toxicity was sharply reduced by limiting the mean scleral temperature to <=44°C. This study employing 30% lower radiation doses, showed tumor regression in the majority of patients. Longer follow-up is needed to assess long-term treatment efficacy and late treatment complications.
Key Words: Episcleral plaque radiotherapy, hyperthermia.This paper was presented at the 80th Scientific Assembly and Annual Meeting of Radiological Society of North America, November 27 to December 2, 1994, Chicago, Illinois.
In the past 20 years episcleral radioactive plaque therapy has become a well-established treatment modality in the management of patients with uveal melanomas (1-5). In a series of 3,000 consecutive patients with this diagnosis treated in Wills Eye Hospital in Philadelphia there has been a sharp increase in the use of episcleral plaque radiotherapy with a corresponding decrease in the number of patients treated with enucleation (1). The 5-year actuarial survival rates of >75% have been reported in episcleral plaque-treated patients (1,4,5,6).
Serious treatment complications occur in >20% of patients treated with episcleral plaque radiotherapy, particularly in those with larger lesions requiring higher radiation doses (2,4,6,7). This includes ∼10% of patients who require enucleation to manage severe postradiation sequelae (4,5). Introduction of treatment optimization program in one study has resulted in a sharp decrease in the incidence of serious postradiation complications (8,9). The remaining problem in a substantial proportion of radioactive plaque-treated patients is a progressive decrease in visual acuity (1,4,8).
In the past several years, attempts have been made to reduce radiation doses in patients treated with episcleral plaque radiotherapy and to compensate for it by adding adjuvant hyperthermia (10-14). This adjuvant hyperthermia was expected to enhance the effectiveness of radioactive plaque therapy and obtain an improvement in long-term visual acuity through radiation dose reduction. In Phase I-II clinical trials, mean radiation doses to tumor apex were reduced from 30 to 50% (15-17). In a report on the treatment of 44 patients managed with episcleral thermoradiotherapy with markedly reduced radiation doses, tumor control rates were similar to those obtained with full radiation doses without hyperthermia (16). Additional benefits included good treatment tolerance and a low incidence of severe postradiation complications (16). This report presents results of a Phase I clinical trial using episcleral plaque thermoradicitherapy with localized current field (LCF) system in patients with posterior uveal melanoma.
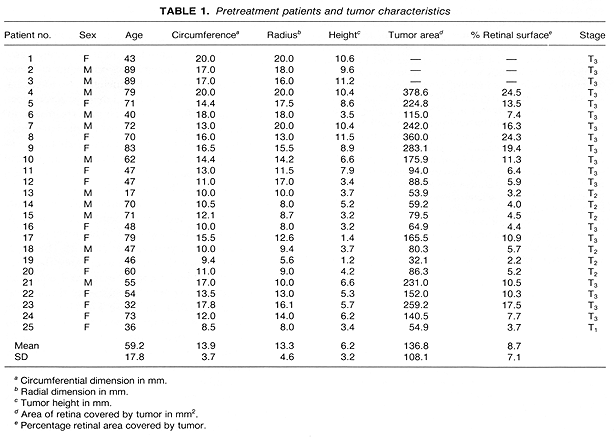
From 1988 to 1991, 25 patients with uveal melanoma were treated with episcleral thermoradicitherapy in a Phase I study at this medical center. There were 14 (56%) female and 11 (44%) male patients. The patients ages ranged from 17 to 89 years (mean, 59 years). Tumor was staged according to the American Joint Committee on Cancer Staging System (18). Most (72%) patients had T3 tumors (Table 1). Tumor dimensions were circumferential, 8.5-20 mm (mean, 13.9 mm) and radial, 5.6-20 mm (mean, 13.3 mm); and the tumor height ranged from 1.4 to 11.5 mm (mean, 6.2 mm).
In 22 study patients, tumor area and the percentage of retinal surface infiltrated by the tumor were calculated. The tumor area ranged from 32.1 to 378.6 mm² (mean, 136.8 mm²) (Table 1). The mean retinal area infiltrated by the tumor was 8.7% (Table 1).
Tumor surface area was estimated with use of a combination of computerized axial tomography imaging and fundus photography. First, ocular dimensions were measured from computerized axial tomography, and a three-dimensional (3D) model of the eye was constructed. The retinal surface was estimated to a portion of a sphere of radius r, extending from the posterior pole to the limbus. Fundus photos were then digitized and scaled to a retinal diagram representation of the model. The dimensions of, and distance between, anatomic landmarks visible in the fundus photos, such as the optic disc and fovea, provided the scaling and orientation factors. The tumor perimeter was then outlined on the fundus diagram and stored as a polygon. The surface area A of an n sided polygon on the surface of a sphere (where A is the area, φ is the sum of its angles in radians and r is the radius of the sphere is A = [φ - (n - 2)π]r². The angles between successive sides of the polygon were calculated and summed, and the surface area calculated.
Detailed pretreatment general and ophthalmic examinations were performed for all patients, and the findings are described elsewhere (5,8,17). Echography was considered an essential part of the initial evaluation of all tumors in this study, as well as for follow-up examinations. Both contact A-scan, and B-scan ultrasound were utilized. The diagnosis of choroidal melanoma was based on acoustic criteria previously described (19). The shape, size, and location of the tumors were all noted and were observed after treatment for indications of either growth or regression, as well as for changes in their internal acoustic characteristics.
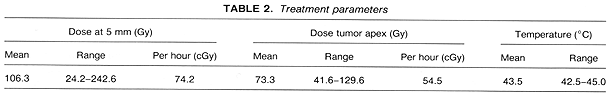
Episcleral plaque radiotherapy was given with use of I-125 in the previously described custom-built gold plaques (5,6,8). The treatment was optimized with use of 3D image reconstruction and 3D radiation dose distribution (9). A mean radiation dose to the tumor apex was 73.3 Gy, given at a mean rate of 54.5 cGy/h (Table 2). Episcleral hyperthermia was delivered with a localized current field system at 500 kHz (12,17). The temperature was controlled on the plaque surface with four microthermocouples at a mean temperature of 43.5°C. This mean temperature was easy to obtain and maintain throughout the treatment. The temperature was controlled within a narrow range of the mean (±0.5°C). This hyperthermia was given once for 45 min at a steady state just before the initiation of brachytherapy (17). The entire episcleral procedure, including plaque placement and removal, was performed on an outpatient basis. Details on surgical techniques as used in this study have been published (5,20). Follow-up ranged from 20 to 68 months (mean, 31.2 months). The details of the follow-up examinations have been published (17). All patients with uveal melanoma considered for this Phase I study refused to be randomly assigned to an appropriate Collaborative Ocular Melanoma Study (COMS) protocol. Informed consent was obtained in writing from all patients.
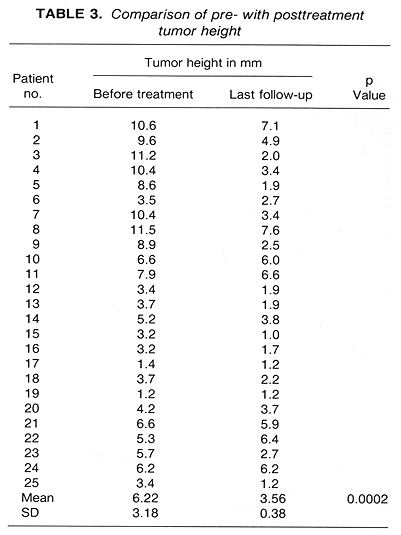
Tumor height reduction was recorded in 22 (88%) patients (Table 3, Fig. 1). A comparison of posttreatment to pretreatment tumor height showed a mean decrease of 2.66 mm or a 43% height reduction, p = 0.0002 (Table 3). Of these 22 patients with tumor height reduction, two had subsequent tumor progression, following initial tumor regression. Patient 21 showed an excellent and rapid tumor regression, from the initial 6.6 mm to 1.4 mm at 4 months. This regression was maintained for 4 months with a subsequent rapid tumor height increase to 5.4 mm taking place within I month. This patient is doing well following an enucleation. Patient 22 was treated with episcleral plaque radiotherapy 6 years before the present study. She showed late tumor recurrence and was re-treated with episcleral plaque thermoradiotherapy. This retreatment failed with tumor progression recorded at the 3-month follow-up examination. The patient is currently doing well following an enucleation. Of the remaining three study patients, Patients 19 and 24, to date, showed no change in tumor height, and Patient 22 showed tumor progression (Table 3).
Metastatic disease was seen in three (12%) patients (Patients 2, 3, and 6) of which two developed metastatic uveal melanoma and one, with the primary tumor in regression, developed a metastatic breast adenocarcinoma. Clinical evidence of their metastatic disease was seen at 14, 16, and 18 months, respectively. Of the three patients with disseminated tumor, two died within 5 months of diagnosis and one remains alive with a rapidly progressing metastatic disease.
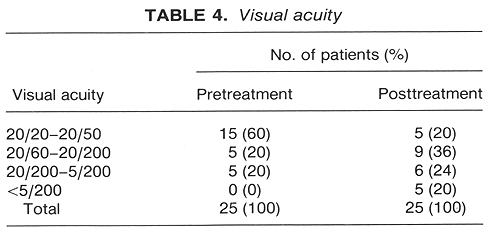
Visual acuity has gradually decreased over the period of observation (Table 4). At the entry to this study there were 60% of patients with visual acuity >=20/50, whereas only 20% maintained this visual acuity at the last follow-up examination (Table 4). In all, 80% of patients maintained at least ambulatory vision (>5/200).
The treatment was well tolerated by the patients. Severe complications were seen in two (8%) patients early in this study. They included vitreous hemorrhage and a large hemorrhagic retinal detachment. These complications were noted within 3 weeks of completion of therapy. They were thought to represent hyperthermia toxicity. A mean controlled temperature reduction to :f~440C resulted in no more severe toxicity being recorded in the remaining study patients. Of the two patients who had severe treatment toxicity, one with a nonfunctioning eye required enucleation. Histological examination of the enucleated eye showed a massive tumor necrosis with a small persistent focus of viable tumor at the periphery of the lesion. Moderate toxicity was seen in two (8%) patients who had retinal detachment requiring no therapy. Cataract developed in five (20%). Mild complications such as temporary diplopia, photophobia, vitreous hemorrhage, and retinal detachment occurred in six (24%) patients.
Episcleral thermoradiotherapy was easy to administer in all patients. Mean temperature >42.5°C was obtained and maintained for 45 min in 24 (96%) patients. In Patient 3, hyperthermia had to be discontinued at 30 min, because of technical problems with temperature sensors.
The outcome of this Phase I clinical trial is in agreement with the findings of our preclinical studies (21). The mean temperature range from 42.5 to 44°C, as controlled on the surface of the plaque, using the instrumentation designed and developed in this medical center, was well tolerated. The hyperthermia effects were limited to the area covered by the plaque. In addition, there was strong evidence of therapeutic activity with a mean radiation dose 30% lower than the one generally prescribed in patients with uveal melanoma treated with episcleral plaque radiotherapy (1,2,6,8). The findings of this study suggest that the addition of LCF episcleral hypertherriia enhances the effectiveness of episcleral plaque radiotherapy in terms of improved tumor response and reduction of postradiation complications. These findings however should be interpreted cautiously as they represent an experience with a relatively small (n = 25) number of patients who were observed for a short period (mean, 31 months). An improvement in the incidence of tumor control and the reduction in treatment complications obtained with the use of adjuvant episcleral hyperthermia should be established in a Phase III prospective randomized trial.
Finger, recently reported on the treatment of 44 patients with uveal melanoma using different thermoradiotherapy instrumentation (16). The author obtained an excellent tumor control rate, good treatment tolerance, and a low incidence of complications with lower radiation doses than those used in the present report.
At the present, we are in the process of conducting a Phase II prospective, randomized trial. This new study is an attempt to search for an optimal radiation dose in patients with uveal melanoma who are treated with episcleral plaque thermoradiotherapy. Should lower radiation doses given with hyperthermia prove equally effective in terms of tumor volume reduction and good survival rates, as higher radiation doses, given without hyperthermia, one could also expect to obtain a lower incidence of severe radiation complications, resulting in an improvement in visual acuity.
In addition to the above study, in order to reduce the incidence of late radiation complications and to increase the likelihood of preservation of useful vision, a major effort is being made at this medical center to further optimize the design of I-125 containing episcleral plaques. The newly designed episcleral plaque is expected to substantially (>=30%) reduce radiation dose to the sclera, while maintaining a tumoricidal dose to the apex of the lesion. This is accomplished through collimation of individual I-125 sources. The modified episcleral plaque is currently being introduced to the clinic.