Between 1983 and 1987, 56 patients with choroidal melanoma were treated at the University of Southern California with episcleral plaque (RPT). There were 29 female and 27 male patients, with a mean age of 59 years. Tumor stage at diagnosis was T2 in 18 (32%) and T3 in 38 (68%) patients. The tumor height ranged from 2.9 to 15 mm (mean 6.8 mm). Radial dimensions ranged from 5 to 25 mm (mean 13.2 mm), and circumference ranged from 7 to 23 turn (mean 12.3 mm). Most (77%) patients had posteriorly located tumors, including 18% that were j uxtapapillary. Custom-designed gold plaques were utilized in this study. Radioactive isotopes used were I-125 for 26 procedures or Ir-192 for 31 procedures. A total of 56 patients were treated, with one patient having two procedures. Radiation doses at the tumor apex ranged from 29.8 to 165.4 Gy (mean 94.5 Gy), with the dose at 5-mm depth ranging from 70.5 to 430 Gy (mean 161.5 Gy). Follow-up ranged from 29 to 57 months (mean 39 months). The overall 4-year survival was 96%, with a 9 1 % incidence of free-ofdisease progression at 4 years. The majority (84%) of patients experienced a decrease in tumor height, with 27 (48%) patients having >50% decrease. Increase in tumor height was noted in 5 (9%) and no change in 4 (7%) patients. Useful vision (>5/200) was observed in 59% of patients, including 21% who had improved vision. Metastatic tumor occurred in 5 (9%) patients, with a mean time to metastases of 14 months. There was a good correlation between radial tumor dimension and metastatic disease, p < 0.00 1. Treatment complications were observed in 34 (61 %) patients, with cataract and retinopathy being the most common. Enucleation was performed in 11 (20%) patients, with a mean time to enucleation of 14.5 months. Causative factors for enucleation were treatment complications in 6 and tumor progression in 5 patients. Enucleations were required primarily in patients with tumors > 8 mm in height (p = 0.009). Improved RPT techniques with three-dimensional dosimetry are needed to reduce the overall incidence of treatment complications. Adjuvant hyperthermia is being investigated in an attempt to improve tumor control in patients with larger tumors.
Radioactive episcleral plaque therapy (RPT) has been commonly applied in the treatment of primary malignant melanoma of the uveal tract (MMUT) since the early 1970s. In 1939, modern brachytherapy techniques were introduced by Stallard, who designed a radioactive cobalt-60 episcleral applicator (1). He also demonstrated the successful use of this applicator in clinical practice (1). Lommatzsch has used a Ru-106/Rh-106 episcleral plaque since 1964 and has reported excellent results in a series of 309 patients (2). In the United States, Shields and Brady of Wills Eye Hospital are responsible for the introduction of cobalt-60 plaque therapy into clinical practice (3,4). Packer and Rotman have popularized the treatment of choroidal melanoma with episcleral plaque containing radioactive I-125 (5,6). Other successful applications of radiotherapy, resulting in a high incidence of tumor control with preservation of eye function, have been reported with the use of proton and helium ion beams (7,8).
The purpose of this report is to present our clinical experience with MMUT using custom-designed I-125 and Ir-192 episcleral plaques.
Between June 1983 and July 1987, 56 patients with MMUT were treated with RPT at the University of Southern California School (USC) of Medicine. There were 29 female and 27 male patients, with a mean age of 59 years (range 23-91 years).
Pretreatment evaluation consisted of a detailed general and ophthalmic history and physical examination. Detailed ophthalmological examination was performed at the USC Doherty Eye Institute. This examination included a general eye examination, visual acuity, biornicroscopy, fundus photography and ultrasono graphic studies. Ultrasound examination was of critical importance in helping to establish a diagnosis of MMUT and in providing accurate tumor measure- Slots for ments (9). All patients were examined by an I-125 seeds ophthalmic ultrasound specialist using A and B scans. Other examinations included radiographs of the chest (posteroanterior and lateral), complete blood count, serum alkaline phosphatase, and serum lactic acid de hydrogenase. Lesions suspected of being metastatic tumor required needle biopsy for histological confirmation.
A clinical staging system, as recommended by the American Joint Committee for Staging of melanoma of the uvea, was used in this study (10). There were 18 (32%) T2 and 38 (68%) T3 patients. Stage grouping recommended by the Collaborative Ocular Melanoma Study (COMS) divides tumors into 3 groups: I-small tumors <= 3 mm in height and up to 5 mm in diameter; II-intermediate tumors 3.1-8 mm in height and/or up to 16 mm in diameter; and III-large tumors > 8 mm in height and/or > 16 mm in diameter.
Utilizing COMS groups, there were no patients with small tumors, 21 (37.5%) had large tumors, and 35 (62.5%) had intermediate sized tumors, including 17 (30%) with tumors >= 18 mm in height. The tumor height ranged from 2.9 to 15 mm (mean 6.8 mm). Initial radial dimensions ranged from 5 to 25 mm (mean 13.2 mm), and the initial circumference ranged from 7 to 23 mm (mean 12.3 mm). The tumor was located posterior to the equator in 43 (77%) patients, including 10 (18%) who had juxtapapillary location and 1 (2%) extending anterior to the equator. Of the 13 (23%) patients with anterior tumors, It (20%) had involvement of the ciliary body and 1 (2%) extended posterior to the equator.
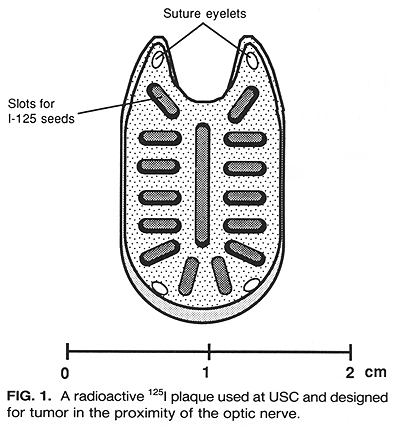
Episcleral plaques used in this study were cast in 18K gold by the lost wax technique, as sections of a spherical shell >= 1.5 mm thick. Grooves for embedding Ir-192 or I-125 seeds were placed radially (Fig. 1). Details on these plaque designs and dosimetry have been published (11,12). Initially, a new plaque was designed for each patient. With time, however, a number of variably sized and shaped plaques have been accumulated to accommodate the demands of almost any clinical situation.
Radioactive plaque placement was performed as an outpatient procedure. Retrobulbar and eyelid block were done to obtain anesthesia and akinesia in the region of interest. Additionally, patients received intravenous sedation prior to the procedure. The conjuctiva was opened and Tenon's capsule dissected off the sclera. Traction sutures were placed under the rectus muscles. The eye was examined with indirect ophthalmoscopy and transillumination. The tumor outline was marked on the sclera with a Mira diathermy. Subsequently, a nonradioactive plaque was placed over the scleral marks, usually giving a 2-mm margin to the visible tumor. This plaque was sutured into the sclera and the eye reexamined to assure proper placement. If the position of the plaque was satisfactory, a radioactive plaque replaced the nonradioactive one. The position of the plaque was again verified, and the conjunctiva was closed with 6-0 plain cut gut sutures. Gentamycin (20 mg) and dexamethosone (40 mg) were injected into the subtenon space. The eye was covered with a patch and an aluminum cover, to which a lead shield was attached. The patient was discharged with appropriate radiation safety instructions. Removal of the implant was also performed on an outpatient basis following administration of the desired dose of radiation.
A total of 57 procedures have been performed in 56 patients. The patient who had 2 plaque placements had the first procedure with I-125 and, 11 months later, had the second procedure with Ir-192 for tumor growth outside of the previously treated volume. Iodine-125 was used in 26 (46%) procedures and iridium-192 in 31 (54%) procedures. Since the summer of 1986, only I-125 plaques have been used for this treatment of patients due to the superior radiation safety of this isotope as compared to Ir-192. Of the 56 patients in this study, 20 (36%) with the largest tumors were treated with RPT because of their refusing enucleation.
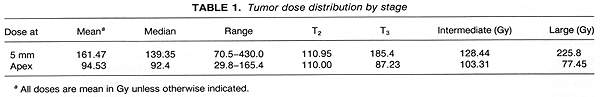
Radiation doses were defined at the tumor apex and at 5-mm depth measured from the outside of the sclera. A margin of tissue 2 min from the base of the lesion was scheduled to receive the same radiation dose as the tumor apex. The total radiation dose to the apex was prescribed to be 80-120 Gy, given over an average period of 7 days. Preliminary analysis of our experience with several patients with large tumors (>10 mm in height) showed frequent treatment complications following the administration of >200 Gy at 5-mm depth. A dose reduction was introduced, with the dose at 5 mm to be <200 Gy. Details on dosimetry problems in the 56 patients treated have been published (11,12). The mean dose delivered at the tumor apex was 94.53 Gy (range 29.80-165.40 Gy). The mean dose at 5mm depth was 161.47 Gy (range 70.50-430.0 Gy). Details on tumor doses are shown in Table 1.
Follow-up examinations were scheduled after removal of the implant at 1, 3, and 6 weeks, and quarterly thereafter. At these regularly scheduled follow-up visits, the patients were to undergo oplithalmologic examination, similar in complexity to the pretreatment examination. Ultrasound examination was performed at quarterly intervals. A regular follow-up schedule was difficult to maintain, as a substantial number of patients were referred from out of the region. The mean followup was 39 months (range 20-57 months).
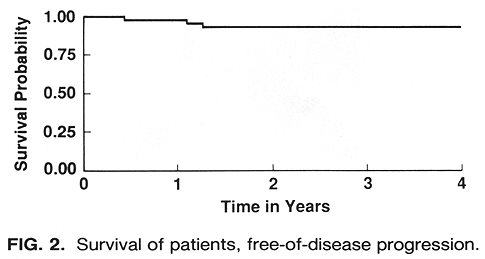
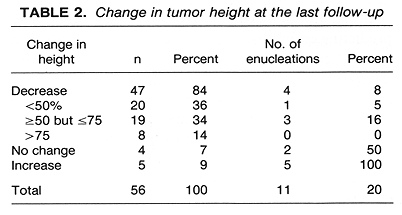
The overall 4-year survival was 96%, and the 4-year survival, free-of-disease progression, was 91 % (Fig. 2). Of the 56 patients treated, 47 (84%) experienced a decrease in tumor height, with 5 (9%) experiencing an increase in tumor height and 4 (7%) no change (Table 2). Complete tumor regression, with only a flat scar remaining, was noted in 4 (7%) patients. In addition to the 47 patients who experienced a decrease in tumor height, 2 patients initially showed a substantial (>70%) decrease in tumor height with a subsequent relapse.
examination and was compared with the pretreatment values. A total of 33 (59%) patients retained useful vision, defined as ~:5/200. Of the 56 treated patients, 12 (2 1 %) experienced improved vision, and in 2 (4%) patients, vision remained unchanged. The patient who had 2 plaque placements had no complications, with >50 tumor height reduction and visual acuity of 20/200 recorded 24 months following the second procedure.
Metastatic disease was observed in 5 (9%) patients. In 4 of these patients, liver was the site of metastases, whereas I patient developed skin metastases. All 5 patients developed clinical signs of metastatic disease within 22 months of treatment (at 5, 12, 15, 17, and 22 months). Of these 5 patients, 3 died of metastatic tumor and 2 remain alive with progressive disease. It is of interest to note that the mean pretreatment radial tumor dimensions of the 5 patients with metastatic disease was 19.6 mm, whereas it was 13.26 min for those without metastatic disease (p < 0.001, Mest). Such a correlation did not exist between the other two tumor dimensions and metastatic disease, although 4 of 5 patients had a tumor height greater than the median.
Treatment complications were observed in 34 (61%) patients. The most common complication was cataract formation, seen in 36% patients, followed by radiation retinopathy (29%) and optic nerve atrophy (20%) (Table 3). There was a good correlation between complication-free status and tumor height (<9 mm) and lower doses of radiation calculated at 5-mm depth (Figs. 3 and 4). There were only 2 patients free of complications among those who received > 150 Gy at 5-mm depth (Fig. 4). There was no evidence of a greater incidence of complications among the 38 T3 patients as compared with the 18 T2 patients (p = 0.36, Fisher's exact test).
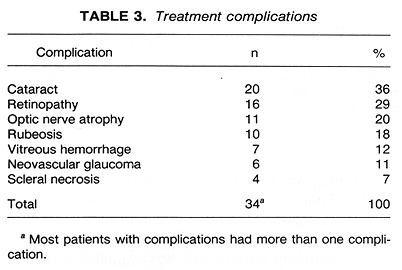
Of the 56 patients treated, 11 (20%) underwent enucleation (Table 2). The mean time from treatment to enucleation was 14.5 months (range 3-27 months). The reason for enucleation in 5 patients was the posttreatment increase in tumor height. The mean time to enucleation for these 5 patients was 15.6 months (range 3-27 months). In 4 patients, enucleation was performed because of severe treatment complications, although there was up to a 75% decrease in tumor height. The remaining 2 patients had no change in tumor height, and enucleation was required by the presence of treatment complications. The mean time to enucleation in the 6 patients enucleated for complications was 13.5 months (range 5-27 months). Histological findings in the 11 enucleated patients were mixed cell melanoma in 5, spindle cell in 2, low-grade melanoma in 1, and 3 patients had extensive necrosis with no tumor cells identified in the specimen. A detailed study of these histological findings is currently underway at USC.
Initial tumor height was an important parameter predicting enucleation (Fig. 3). Of the 24 patients with tumor height <=6 mm, 2 (8%) had enucleation. This compared with 4 (18%) enucleations in the 22 patients who had tumor height > 6 but <= 10 min and 5 (50%) enucleations among the 10 patients with tumor height > 10 mm (p = 0.009, likelihood ratio test for trend). There was no such correlation among the initial radial dimension, tumor circumference, and enucleation (p = 0.53).
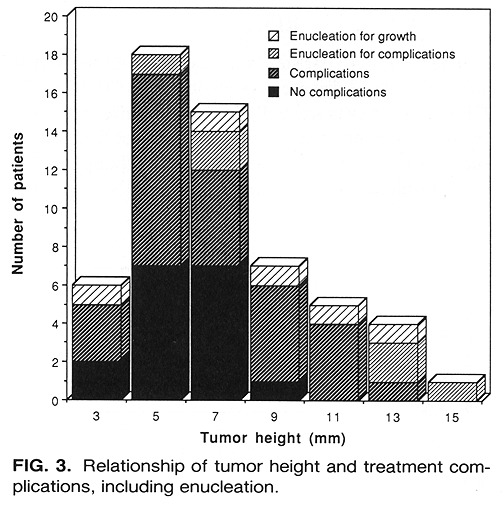
There was a higher incidence of enucleations among the 38 T3 patients as compared to the 18 T2 patients (24% versus 11%, p = 0.47, Fisher's exact test). Of the 11 enucleated patients, 9 had T3 tumors. The incidence of enucleation among intermediate versus large tumors by COMS groups was 5 (14%) versus 6 (29%) patients (p = 0.009). There was also a difference in the incidence of enucleation between these patient groups (p = 0.009). The incidence of enucleation in patients treated with I-125 as compared to those treated with Ir-192 was 8% versus 30% (p = 0.05, Fisher's exact test). This difference, however, should be interpreted cautiously due to a greater mean tumor height of the Ir-192 treated patients (7.4 versus 6.0 mm) and a longer mean period of observation (7 months) as compared to the I-125 treated group.
The mean radiation dose at 5-min depth in the 45 nonenucleated patients was 149 Gy, and it was 218.5 Gy in the 11 enucleated patients (p = 0.02, t test for equal mean dose) (Fig. 4). The incidence of enucleation by radiation dose to the tumor apex was 23, 17, and 20% forthe patients receiving <80, >=80 but <=100, and >100 Gy, respectively (not significant).
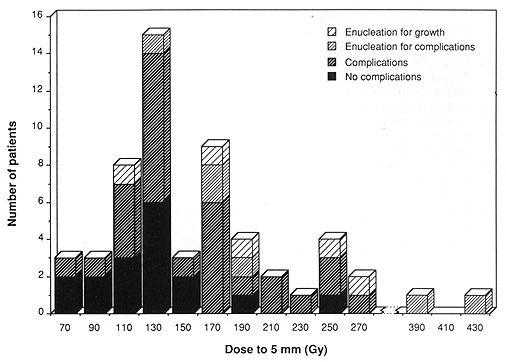
Fig. 4. Distribution of patients by radiation dose to the depth of 5 mm and its relationship to treatment complications, including enucleation.
This study shows a 61% incidence of treatment complications. Approximately one-half of these patients had complications that could be effectively managed, as in the case of a surgical removal of a formed cataract. In six patients, however, the complications were of sufficient severity to require enucleation, in spite of substantial tumor regression in four of these patients. An additional five patients required enucleation for tumor progression. The lack of tumor response or tumor growth tends to occur early. The mean time to enucleation was 15 months, and objective evidence of progression occurred a few months earlier. Similarly, most serious complications occurred within the first 18 months of therapy.
Clinically important radiation injury of the eye has been reported to occur in approximately 30% of the patients treated with teletherapy for tumors in the proximity of the globe (13,14). The radiation dose required for this incidence of complications was >60 Gy. In patients with ocular melanoma treated with RPT, the incidence of clinically important complications was reported to be up to 40% (1,3,6,15). Complications requiring enucleation occurred in approximately 10% of patients treated with RPT (1).
In the present study, the incidence of treatment complications is higher than generally reported. There are several possible explanations for this. Of the 56 patients treated, 38 (68%) had T3 disease. This included 17 (30%) patients with tumor elevation >= 8 mm and a radial dimension > 16 mm in 7 patients. An additional 8 (14%) patients had > 16 mm radial dimension with tumor height < 8 mm. The above 25 (45%) patients were candidates for enncleation, but they refused surgical treatment. Several of these patients maintained useful vision.
At the present time, major efforts are being made to optimize RPT. These efforts are directed toward a routine use of radioactive plaques containing I-125 rather than Co-60 or Ir-192, better understanding of I-125 dosimetry, and a wide utilization of three-dimensional dosimetry for pretreatment planning (11, 12,16). These efforts may result in reduction of radiation dose to the uninvolved ocular structures, which in turn may help to reduce the incidence of late treatment complications.
Treatment with RPT of large (>8 mm, in height) choroidal melanomas remains a major problem. Radiation dose required for tumor control is likely to result in serious late treatment sequelae. On the other hand, effective treatment for these large lesions, consisting of either enucleation alone or in combination with teletherapy, results in a loss of eye function. The fear of loss of eyesight is a frequent reason for refusal of surgical treatment. The use of adjuvant hyperthermia (HT) to increase the incidence of tumor control in these large lesions is particularly appealing and needs to be investigated in clinical trials (17-19).