Episcleral Plaque Thermoradiotherapy in Patients with Choroidal Melanoma
Int. J Radiation Oncology Biol Phys., Vol. 23, pp. 599-603 Copyright 1992 Pergamon Press Ltd.
Zbigniew Petrovich,¹ M.D., Melvin A. Astrahan,¹ PH.D., Gary Luxton,¹ PH.D., Ronald Green,² M.D., Bryan Langholz,³ PH.D. and Peter Liggett,² M.D.
1. Dept. of Radiation Oncology.
2. Dept. of Ophthalmology.
3. Dept. of Preventive Medicine.
University of Southern California, School of Medicine, 1441 Eastlake Ave., Los Angeles, CA 90033.
ABSTRACT
From 1988 to 1991, 21 patients with uveal melanoma were treated in a Phase I study with episcleral plaque radiotherapy (EPRT). This irradiation was combined with localized current field episcleral hyperthermia (LCFHT). Tumor stage was: T3 = 15 (71%) and T2 = 6 (29%). Follow-up ranged from 2 to 42 months (mean 9.2 months). EPRT was given using custom built I-125 gold plaques. Radiation doses to the tumor apex ranged from 13 to 123 Gy (mean dose 70.0 Gy) given at a mean dose rate of 55 cGy/hr. LCFHT was given with 500 KHz frequency for 45 min immediately before EPRT. The temperature was controlled on the scleral surface using four thermocouples. T mean ranged from 42.5°C to 45°C ± 0.5°C (mean 43.4°C). The study patients showed rapid tumor necrosis. A 25% mean decrease of apical tumor dimension was noted, p = 0.0007. At least ambulatory vision (> 5/200) was maintained by 17/21 (81%) patients. Visual acuity was seen to improve > 6 months post-plaque therapy in 10 (48%) study patients. This was following an intermediate decrease in visual acuity. Severe complications, including large hemorrhagic retinal detachment and large vitreous hemorrhage, were seen in two (9.5%) of the early study patients. A mean scleral temperature reduction to <= 44°C ± 0.5°C resulted in good treatment tolerance and a lack of serious complications in subsequently treated patients. A Phase II prospective randomized trial comparing LCFHT with 60 versus 80 Gy EPRT dose to the tumor apex is currently being activated for patients with choroidal melanoma.
Key words: Choroidal melanoma, Hyperthermia, Radiotherapy.
Accepted for publication 2 January 1992.
INTRODUCTION
Episcleral plaque radiotherapy (EPRT) is a well-recognized, effective treatment for T1, T2, and selected T3 choroidal melanoma patients (4,5,13,15,16). Treatment results in this conservatively treated group are currently being compared with enucleation in a multi-institutional, prospective randomized trial conducted by the Cooperative Ocular Melanoma Study (COMS). This important study requires several more years of patient accrual before the final outcome can be fully analyzed. A relatively slow accrual into the COMS trial is largely because of the patients' reluctance to be randomized into a study where half of the subjects are to undergo enucleation.
Episcleral irradiation is an efficient treatment with survival, commonly reported in over 75% of patients (5,11,13,16). In patients who present with tumors larger than 8 mm in elevation and/or > 15 mm in basal dimension, higher radiation doses are needed to obtain tumor control. This, in turn, results in a sharp increase in the incidence of serious radiation injury which leads to decreased visual acuity (VA) and may lead to enucleation (7,11,13). Very poor VA or the need for enucleation diminishes the attractiveness of conservative treatment in these patients.
Ocular hyperthermia (HT) with microwaves or ultrasound has been under laboratory investigation and used in clinical studies in patients with large tumors since the early 1980's (6,8,10,17). In the reported studies employing HT, substantially lower doses of EPRT were used than in those patients who were treated with EPRT alone (6,9). In spite of the lower radiation doses used in these studies, a high incidence of tumor control was noted together with a low incidence of serious complications (6,9).
The purpose of this report is to present our clinical experience with EPRT-LCFHT combination in patients with choroidal melanoma.
METHODS AND MATERIALS
Between 1988 and 1991, 21 patients with choroidal melanoma were treated in a Phase I study at the Departments of Ophthalmology and Radiation Oncology, University of Southern California School of Medicine. The treatment consisted of EPRT-LCFHT combination. There were 11 (52%) male and 10 (48%) female patients. Their ages ranged from 17 to 89 years with a mean age of 61 years. Tumor stage, using the American Joint Committee Staging System, was T2 in six patients (29%) and T3 in 15 (71%) patients (1). Initially during this study, only patients with very large tumors were accepted for the treatment (Table 1). Following the entry of the first 10 patients, less advanced tumors were also considered for admission to the study. Full vision, permitting reading or driving (>= 20/50), was seen in 12 (57%) while six (29%) were able to read large print only (> 20/50 to >= 20/200). The three (14%) remaining patients had very poor visual acuity (Table 1).
Tumor elevation ranged from 1.2 to 11.5 mm with a mean elevation of 6.4 mm. The mean tumor circumference and diameter were 13.5 and 12.6 mm, respectively (Table 1).
Pre-treatment evaluation included a detailed general and ophthalmic history and physical examination. Ophthalmologic examination was performed at the USC Estelle Doheny Eye Institute by a single ophthalmologist (P.E.L.). This examination included general eye examination, fundus photography, biomicroscopy, and VA. A detailed ultrasound (US) examination was performed on all patients using A and B modes. These studies provided valuable diagnostic assistance and accurate tumor measurements. US of the eye was performed by a single ophthalmic specialist (R.G.). Computer axial tomography of the treated eye was performed to assist in optimization of the radiation treatment plan. General work-up was directed toward the exclusion of possible metastatic disease. Routine laboratory and radiographic studies included: complete blood count, serum alkaline phosphatase and acid dehydrogenase, radiography of the chest, and an electrocardiogram. Suspicion of metastatic disease had to be confirmed by fine needle aspiration biopsy.
Eligible patients had to first be considered for inclusion in the COMS trials. These patients who either were not eligible for COMS protocols or refused randomization were considered for the present Phase I study.
Radioactive and hyperthermia plaques were placed and removed as outpatient procedures. Details of the surgical technique have been published elsewhere (11,16).
Radiotherapy
Each study patient had radiation dose distribution preplanned allowing for dose optimization and the dose reduction to the critical structures of the treated eye. Custom built gold plaques with I-125 were utilized (11,14,16). The total isotope activity ranged from 17.2 to 60.9 mCi. To optimize the treatment, I-125 seeds of 2 to 3 different activities were used (3). Radiation dose was prescribed to D5 mm and to the tumor apex. D5 mm ranged from 24.2 to 242.6 Gy (mean 113.4 Gy) given at a mean dose rate of 76.6 cGy (Table 2). Dose to the tumor apex ranged from 41.6 to 129.5 Gy (mean 75.6 Gy) given at a mean dose rate of 55 cGy/hr (Table 2). The mean duration of radioactive implant was 145.3 hr (Table 2).
LCFHT
A USC designed and built 500 KHz radiofrequency system was used for the treatment of all patients (Fig. 1) (2). A gold plaque used for HT was insulated to direct power deposition to the tumor (Fig. 2). Microthermocouples consisting of four sensors, spaced 2 mm apart, were designed to measure the temperature on the surface of the plaque. The temperature was measured throughout each treatment session. T mean at steady state ranged from 42.5°C to 45°C ± 0.5°C. The average mean temperature was 43.4°C ± 0.5°C. Patients received a single LCFHT session given immediately prior to EPRT. Each session was scheduled for 45 min with an additional 10 min to reach the desired treatment temperature.
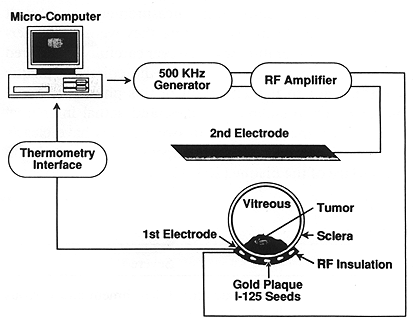
Fig. 1. A diagram of localized current field hyperthermia system as used in this study. When the apparatus is activated, there is a current flow between the gold plaque and the second electrode. Due to the difference in size between the two electrodes, a greater heat deposition is expected to take place in front of the gold plaque, thus heating the tumor. Temperature is regulated by the computer via feedback from microthermocouples on the plaque surface.
Follow-up extended from 2 to 42 months with a mean of 9.2 months. Follow-up visits were scheduled at 2, 6, and 12 weeks post treatment, quarterly during the first post-treatment year and twice a year thereafter. At each follow-up visit, interval detailed general and ophthalmic histories were obtained. Ophthalmological examinations similar to those obtained in the pre-treatment evaluation were performed. US examination was performed initially at the 3 month posttreatment visit. The reason for this was that the tumor measurements performed sooner were found to be difficult to interpret. Informed consent was obtained in writing from all study patients and in two additional patients in whom interstitial temperatures were measured.
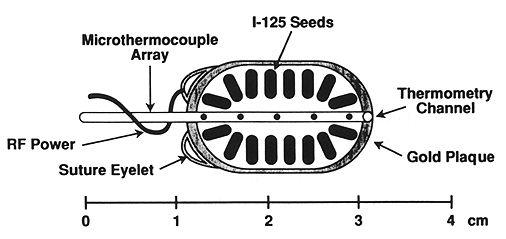
Fig. 2. A schematic representation of an episcleral plaque for localized current field hyperthermia and episcleral plaque radiotherapy.
RESULTS
Therapeutic temperature (>= 42.5°C was easy to achieve and maintain. The mean controlled temperature of 45°C, as obtained in Patient 1, and T mean of 44°C in Patient 2, resulted in rapid and massive tumor necrosis. This was associated with extensive hemorrhagic retinal detachment and extensive vitreous hemorrhage. The findings in both of these patients indicated a higher than expected tumor and normal ocular tissue temperatures. To measure the ocular temperature interstitially, two patients with large T3 choroidal melanomas, scheduled for enucleation, were selected for this procedure. Both of these patients received a pre-operative course of external beam radiotherapy to the ipsilateral orbit consisting of 20.0 Gy given in five equal daily fractions of 4.0 Gy each. From 4 to 48 hr following completion of irradiation, just before enucleation, intraocular temperatures were measured with microthermocouples during LCFHT. The T mean in both cases at the tumor apex was 44°C. In the subsequently treated study patients, the control temperatures with plaques > 20 mm in the largest diameter was 43°C ± 0.5°C, and with smaller plaques < 16 mm in the largest diameter, it was 44°C ± 0.5°C. Since this policy was adopted, no patients with severe toxicity have been observed (Table 3).
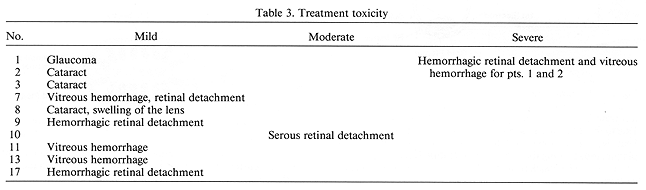
Severe toxicity was seen in two (9.5%), moderate in one (5%) and mild in 10 (48%) patients. Among the patients with mild complications, six had vitreous hemorrhage or hemorrhagic retinal detachment. Both of these conditions appeared early in the post-treatment course and resolved spontaneously over a period of 3-4 months.
A decrease in tumor elevation was evident at 3-month, post-treatment follow-up in nearly all patients. This decrease was noted to continue during subsequent followup visits. A 25% mean decrease in tumor elevation was noted in the study patients, p = 0.0007 (paired T-test). A decrease of > 50% in tumor elevation was seen in four (19%), > 25% < 50% in six (29%), and < 25% in four (19%) patients. The remaining seven (33%) patients showed no change in tumor elevation.
Examination of VA in the immediate post-treatment period (<= 3 mos.) showed a decrease in VA in 16 (76%), an increase in three (14%) and no change in VA in two (9.5%) patients. VA gradually improved and at 6 months post-treatment, 17 (81%) patients had ambulatory vision, including five (24%) who had normal reading vision. Of the 21 patients treated, two (9.5%) are known to have died of metastatic choroidal melanoma. Patient 1 had an enucleation at 16 months post-therapy for treatment complications resulting in a non-functioning eye. This patient developed rapidly progressive metastatic disease to the lymph nodes in the neck and to the liver and died 3 months later of his disease. Patient 2 died of metastatic melanoma at 21 months post-treatment.
DISCUSSION
This Phase I study has shown that episcleral HT-RT combination can be delivered safely in patients with large choroidal melanomas. Hyperthermic temperatures (> 42°C) were easy to achieve and easy to maintain. Temperature gradient on the surface of the episcleral plaque was less than I°C and most frequently within ± 0.5°C. This factor helped to assure a more uniform heat distribution throughout the volume of interest. Higher than expected intraocular temperatures were related to the diameter of the episcleral plaques. In the pre-clinical studies, smaller hyperthermia plaques were used, which resulted in a mean radial temperature gradient of 0.3°C/mm (2,12). The larger plaques, as used in the clinical study, showed a smaller radial gradient, which amounted to a mean of <= 0.2°C/mm. Following the change of the hyperthermia prescription, lowering the controlled temperature for larger plaques to 43°C and for the smaller plaques to 44°C, no more acute toxicity of unusual severity was noted.
At the outset of this study, there was a concern regarding a lack of intratumoral temperature measurements. We felt that invasive temperature measurements may result in serious complications therefore they were not undertaken. The temperature was however carefully monitored using multiple sensors placed on the episcleral plaque surface. Prior to the activation of this phase I protocol our extensive pre-clinical studies, and actual interstitial temperature measurements in two patients have clearly demonstrated the maximum temperature always to be on the surface of the plaque (2,3,12). These data convinced us of a low probability of "hot spots" occurring within the treated eye.
It is of interest to note the clinical course of Patient 1. This patient with a large primary tumor developed severe treatment toxicity and marginal tumor recurrence. Nonfunctioning eye was enucleated 16 months post-therapy. The subsequent clinical course was manifested by metastatic disease in the neck and liver with rapid tumor progression. This lead to the patient's death 3 months later.
A reduction in tumor elevation, as observed in this study, was most likely closely related to the duration of follow-up. The patients with longer follow-up showed progressively better tumor regression. Similarly, VA initially showed a decrease which was very likely related to the presence of acute treatment sequelae. Following a resolution of retinal detachment or vitreous hemorrhage, there was a frequently striking improvement in VA. One of the most gratifying outcomes of this study was the relatively low dose of radiation delivered to the tumor apex. This dose resulted in a greater degree of tumor necrosis than that seen in patients treated at USC with 30 to 50% higher apical doses administered without HT. Radiation dose to the tumor apex, as recommended by COMS, is 100 Gy. A similar dose schedule has been used for the treatment of patients with choroidal melanoma in this institution. Apparently, a mean apical dose of 70 Gy with HT resulted in a greater degree of tumor necrosis than doses in excess of 100 Gy without HT. Similar data have been reported by Finger et al. (9).
Recently at USC we have activated a Phase II prospective randomized trial for patients with choroidal melanoma. The patients are to receive episcleral LCFHT similar to that used in the present report. They will be randomly assigned to groups receiving 60 Gy versus 80 Gy to the tumor apex. Patient accrual for this proposed study is expected to be completed within 36 months. At the same time, an effort is being made to further optimize radiation dose distribution, which is likely to result in the lowering of unnecessary radiation doses to uninvolved important parts of the eye.
REFERENCES
- American Joint Committee on Cancer. Melanoma of the uvea. In: Behrs, O. H., ed. Manual for staging of cancer. Philadelphia, PA: J. P. Lippincott Co.; 1988: 231-233.
- Astrahan, M. A.; Liggett, P. E.; Luxton, G.; Petrovich, Z. A 500 KHz localized current field hyperthermia system for use with ophthalmic plaque radiotherapy. Int. J. Hyperther. 3:423-432;1987.
- Astrahan, M. A.; Luxton, G.; Jozsef, G.; Kampp, T. D.; Liggett, P. E.; Sapozink, M. D.; Petrovich, Z. An interactive treatment planning system for ophthalmic plaque therapy. Int. J. Radiat. Oncol. Biol. Phys. 18: 679-687; 1990.
- Augsburger, J. J.; Gamel, J. W.; Sardi, V. F.; Greenberg, R. A.; Shields, J. A.; Brady, L. W. Enucleation vs. cobalt plaque radiotherapy for malignant melanomas of the choroid and ciliary body. Arch. Ophthalmol. 104: 655-661; 1986.
- Brady, L. W.; Markoe, A. M.; Amendola, B. E.; Karlsson U. L.; Micaily, B.; Shields, J. A.; Augsburger, J. J. The treatment of primary intraocular malignancy. Int. J. Radiat. Oncol. Biol. Phys. 15: 1355-1361; 1988.
- Coleman, D. J.; Lizzi, F. L.; Burgess, S. E. P.; Silverman, R. H.; Smith, M. E.; Driller, J.; Rosado, A. Ultrasonic hyperthermia and radiation in the management of intraocular malignant melanoma. Am. J. Ophthalmol. 101: 635-642; 1986.
- Kindy-Degnan, N. A.; Char, J. R.; Kroll, S.; Stone, R. D.; Quivey, J. M.; Phillips, T. L.; Irvine, A. R. Effect of various doses of radiation for uveal melanoma on regression visual acuity, complications and survival. Am. J. Ophthalmol. 107: 114-120;1989.
- Finger, P. T.; Packer, S.; Svitra, P. P.; Paglione, R. W.; Anderson, L. L.; Kim, J. H.; Jakobiec, F. A. Thermoradiotherapy for intraocular tumors. Arch. Ophthalmol. 103: 1574-1620;1985.
- Finger, P. T.; Packer, S.; Paglione, R. W.; Gatz, J. F.; Ho, T. K.; Bosworth, J. L. Thermoradiotherapy of choroidal melanoma. Clinical experience. Ophthalmol. 96: 13841388;1989.
- Lagendijk, J. J. W. A microwave heating technique for the hyperthermic treatment of tumors of the eye, especially retinoblastoma. Phys. Med. Biol. 27: 1313-1324; 1982.
- Lean, E. K.; Cohen, D.; Liggett, P. E.; Luxton, G.; Langholz, B.; Hyden, E.; Green, R.; Astrahan, M.; Petrovich, Z. Episcleral radioactive plaque therapy: initial clinical experience with 56 patients. AJCO 13: 185-190; 1990.
- Liggett, P. E.; Pince, K. J.; Astrahan, M. A.; Rao, N.; Petrovich, Z. Localized current field hyperthermia: effects on normal ocular tissue. Int. J. Hyperther. 6: 517-527; 1990.
- Lommatzsch, P. K. Results after B-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years experience. Br. J. Ophthalmol. 70: 844-851; 1986.
- Luxton, G.; Astrahan, M. A.; Findley, D. O.; Petrovich, Z. Measurement of dose rate from exposure-calibrated 1- 12 5 seeds. Int. J. Radiat. Oncol. Biol. Phys. 18: 1199-1207; 1990.
- Packer, S. Iodine-125 radiation of posterior uveal melanoma. Ophthalmol. 94: 1621-1626; 1987.
- Petrovich, Z.; Liggett, P. E.; Luxton, G.; Lean, E.; Langholz, B.; Astrahan, M. A. Radioactive plaque therapy in the management of primary malignant ocular melanoma: an overview. Endocurieth. Hyperth. Oncol. 6: 131-141; 1990.
- Riedel, K. G.; Svitra, P. P.; Seddon, J. M.; Albert, D. M.; Gragoudas, E. S.; Koehler, A. M.; Coleman, J.; Torpey, J.; Lizzi, F. L.; Driller, J. Proton beam irradiation and hyperthermia. Effects on experimental choroidal melanoma. Arch. Ophthalmol. 103: 1862-1869; 1985.
Plaque Simulator References |
Guide Contents




